Energy is the backbone of all industrialisation thus, the life blood of every societies. The discovery of radioactivity by Becquerel in 1896 Marked the beginning of the study of atomic structure. In 1919 when Rutherford announced the transmutation of nitrogen, Cockroft and Walter succeeded in splitting the atom in 1932 and this marked a turning point in the exploitation of energy contained in the nucleus.
It is an accepted fact that radiation is injurious to man. Since the earliest days of nuclear power, it was recognized that nuclear waste was very dangerous and that their disposal is a consequential problem. This has aroused a lot of anxiety in the minds of the public as to the possibility of containing the nuclear wastes as nuclear power program expands.
There are different levels of radioactivity associated with nuclear wastes. These are the high, the intermediate and the low level wastes. The low level wastes such as the gaseous effluent and water from cooling ponds, are treated and discharged into the sea or directly into the atmosphere. In some cases they are buried in shales, earth ponds or in shallow trenches. These methods of waste disposal for low level wastes are considered to be harmless since some of the fission products have very short half lives and decay into stable non radioactive materials while they are still in the reactor.
Some have half lives short enough that they can be dealt with in the reprocessing plant as they become harmless. Solid wastes are buried on land or are disposed off by sea dumping. The intermediate level wastes which include irradiated fuel clodding, reactor components and chemical residues are buried in concrete stores and deep trenches.
For high level wastes consisting of fission fragments from reprocessed nuclear fuel, it is first submerged in special water tank, called cooling pond for some years to enable the radioactivity die down. After this the fuel is then reprocessed. Uranium and plutonium are separated from each other and the resulting fission products are compacted and stored as a special liquid in special double walled Stainless Steel tanks. These tanks are further cooled concentrated and fused into a glass material in Stainless Steel until the radioactivity has died away then the containers will be buried until the remaining activity has decayed.
NUCLEAR FUEL CHAIN
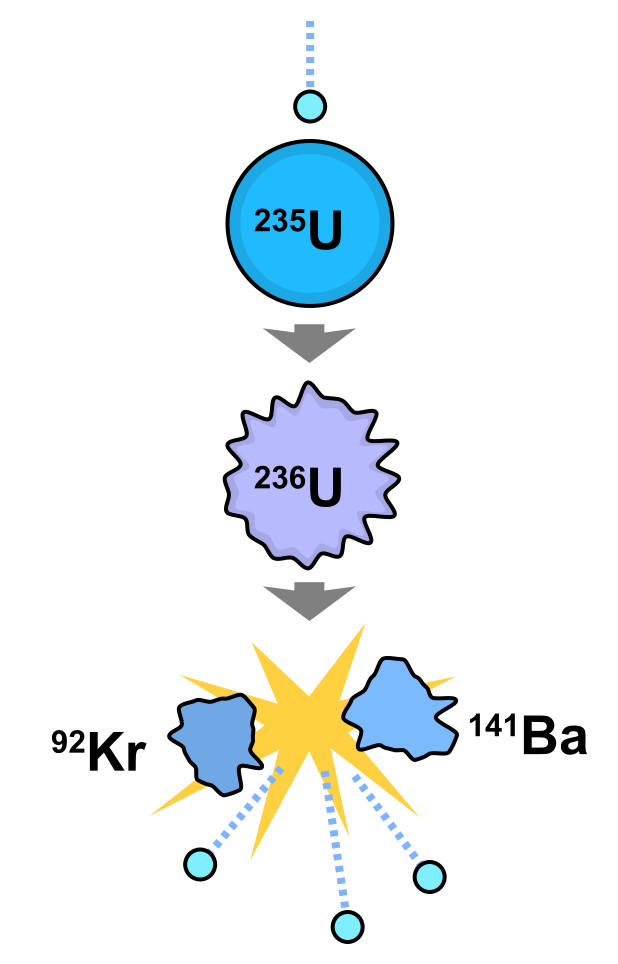
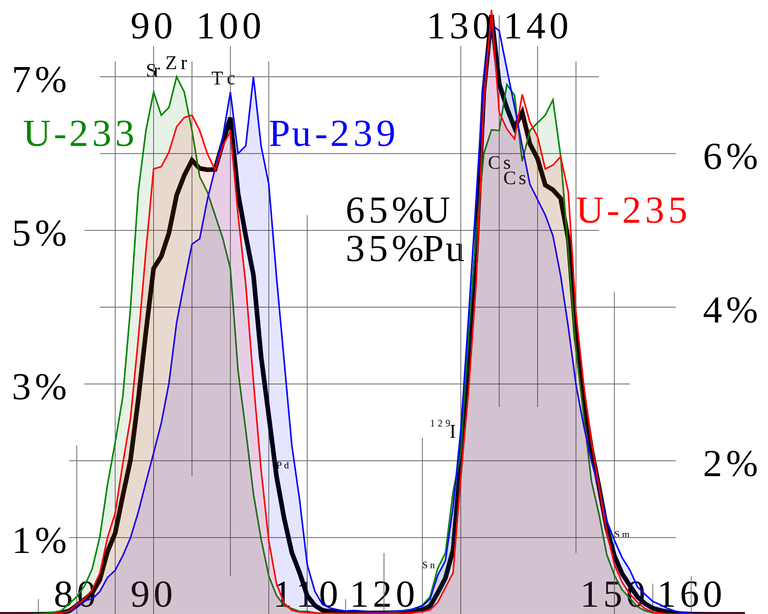
Most natural occurring uranium ore contain less than 1% of uranium in form of uranium oxide. In some cases they contain less than 0.4% of uranium when it is extracted by surface or deep underground mining. Natural uranium contains more than 99% of U-238 and only 0.7% of the fissible isotope U-235 is very useful in the operation of thermal neutron reactors. The usefulness of U-235 stems for the fact that it has a large fission cross-section for thermal neutrons. For U-238 there is a threshold neutron energy of about 1MeV before the isotope can be fissioned.
And this then implies that natural uranium must be artificially enriched by U-235 in order to reduce the size of the reactor core. And this process of increasing the quantity of U-235 in natural uranium is called enrichment. In this process, uranium ore after mining, is milled, concentrated and converted to the volatile gas, uranium haxafluoride (UF6). UF6 Gas is then passed through an enrichment process through a gaseous diffusion plant. Having achieved the desired enrichment that UF6 now enriched in U-235 is converted to either the metal, oxide or carbide and fabricated to the required shape and size. After the fabrication process, the material is then clad in the material cladding, usually Stainless Steel tubes ready for use in the reactor.
After using the fuel in the reactor for a while greater than the run up time, the quantity of U-235 is depleted and it drops below the quantity required to maintain the reactor critical. The fuel is then removed and transported to a chemical processing plant where the fission product U-238 is separated from the remaining quantity of U-235.
ADVANTAGES OF NUCLEAR ENERGY
- Fission products are used in radiation sterilization of food and hospital equipments, in polymer chemistry for cross linking in the manufacture of nuclear batteries and in industrial radiography for the radiographic examination of casting and welded structures.
- The burning of fossil fuels account for an important part of industrial release of green house gases. The annual burning of fossil fuels emits about 20 billions tons of CO2 while a nuclear power station produces electricity without releasing CO2 or any other gases.
- Radioactive materials produced in nuclear installations are also very useful in zoological studies for studying the movement and distribution of insect also in botanical studies in insect population control, determination of the spread of fungicide by farm machinery in plant breading.
REFERENCES
Radioactive waste management
Nuclear fission or nuclear technology
Nuclear transmutation
Fission, Fusion and Nuclear Waste

@chidiebere you were flagged by a worthless gang of trolls, so, I gave you an upvote to counteract it! Enjoy!!