Winter smogs and summer smogs: People have become too reliant on the car. In the UK, for example, nearly a quarter of all car trips are less than 2 miles in length. Of all the journeys people make of between 1 and 2 miles, by any means, 60 per cent are done by car and only 6 per cent by bus, yet 60 years ago bus and coach travel were far more important than travel by car. As a consequence of car travel people now face two types of major air pollution event: winter smogs and summer smogs. Winter smogs happen on cold, calm days when a ‘lid’ of cold air, less than 100 metres from the ground, traps pollutants such as nitrogen oxides, carbon monoxide and sulphur oxides. This happens in the major towns and cities and during these episodes more deaths than usual are recorded.

In the summer, on very hot, sunny days, chemical reactions in the atmosphere lead to the production of low-level ozone. The first two weeks in August 2003 saw the hottest temperatures ever recorded and more than 600 premature deaths were thought to have occurred in the UK as a direct result of increased ozone levels. A major feature of summer smogs is that they may start in urban centres, but recirculating air over Europe, or even from Africa, can sweep in low-level ozone from thousands of miles away.
The amount of carbon dioxide in the atmosphere is increasing and the burning of petrol in cars is a major contributor to this increase. Chemists and engineers can do only so much to reduce car emissions. So people must somehow be weaned from their addiction to the car if the present trend towards serious global warming and climate change, with its alarming consequences, is to be stopped.
ALKANES
Petrol and diesel, the most common fuels for road transportation, are both mixtures that consist mainly of alkane hydrocarbons. This energy comes from when these fuels are burnt in air, and shows that petrol and diesel are both concentrated energy sources. Both fuels are the products of the fractional distillation of crude oil. In this chapter, I will look more closely at these two fuels and the properties of the alkanes that they contain. I will also look at the environmental consequences of using petrol and diesel. So, let’s start by naming the alkanes.
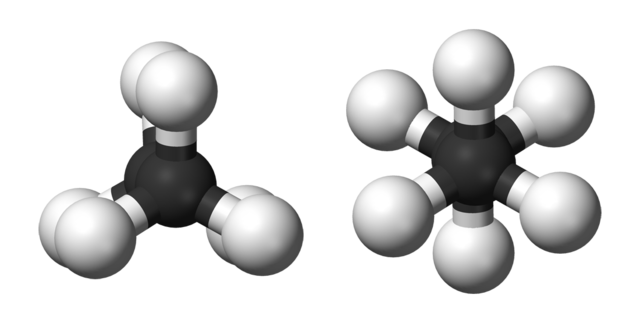
Ball-and-stick models of the two rotamers of ethane. Benjah-bmm27, Public Domain
NAMING ALKANES
Alkanes are saturated hydrocarbons. Remember that saturated hydrocarbons have no double or triple bonds. The carbon atoms are bonded to the maximum possible number of hydrogen atoms. The term ‘saturated’ refers to a time when chemists added hydrogen to various organic compounds and these that would not take up extra hydrogen were called saturated. Around each carbon atom are four σ bonds (sigma bonds) to hydrogen atoms or other carbon atoms. Alkanes could be represented by the general formula CnH2n+2. The table below lists the first six straight-chain alkanes.
Past four, the number of carbon atoms in a molecule is given by a prefix derived from either the Latin or Greek word for the number. For example, in pentane, pent tells you that there are five carbon atoms, and ane tells you that the compound is saturated. (The suffix ‘ane’ was coined by August Hofmann, an eminent German chemist.)
The molecular formulas, the structural formulae and names of the first ten straight-chain alkanes
| Molecular formula | Structural formula | Name |
| CH4 | CH4 | methane |
| C2H6 | CH3 CH3 | ethane |
| C3H8 | CH3 CH2 CH3 | propane |
| C4H10 | CH2 CH2 CH2 CH3 | butane |
| C5H12 | CH2 CH2 CH2 CH2 CH3 | pentane |
| C6H14 | CH3 CH2 CH2 CH2 CH2CH3 | hexane |
| C7H16 | CH3 CH2 CH2 CH2 CH2 CH2 CH3 | heptane |
| C8H18 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | octane |
| C9H20 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | nonane |
| C10H22 | CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 | decane |
Trying this with nonane. The “non” is from the
Latin word for nine and “ane” denotes a saturated molecule. So the molecular
formula is C9H20. Another word you will see for the
system of naming compounds is nomenclature. Naming straight-chain
alkanes is thus straightforward, but what about branched chains? Look at the table
below, that shows some alkyl groups. Alkyl groups are alkanes with one hydrogen
removed so that they can bond with other atoms.
The names and structural formulae of some alkyl groups.
| Name | Structural formula |
| Methyl | CH3 – |
| Ethyl | CH3CH2 – |
| Propyl | CH3CH2CH2 – |
| Butyl | CH3CH2CH2CH2 – |
The name of this branched-chain alkane is
methylpropane:
The longer carbon chain has three carbons (hence propane) and a methyl side group. Sometimes, the molecule is called 2-methylpropane to show that the methyl group is attached to the second carbon in the propane chain. But in the case of this molecule, the 2- is usually left out, as this is the only place the methyl group can go. In the structural formula of 2-methylbutane. Note that the carbon atoms in the longer chain are numbered so that the position of attachment of the alkyl group is the lowest number possible, 2.
EXAMPLE
The compound shown in the figure below used to be called iso-octane, It is important in working out the octane numbers of fuels. What is its systematic name?
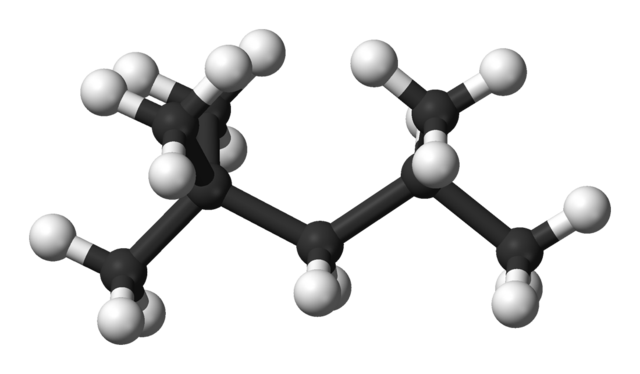
Ball and stick model of iso-octane. Ben Mills, Public Domain
ANSWER: Step 1. Look for the longest carbon chain (called the parent alkane) and name it. In this case, the chain has five carbon atoms, so it will be called pentane.
Step 2. Name every side group. The methyl group is the only side group, but there are three of them. We must ensure the name includes all the side groups, so we use tri, and hence trimethylpentane, If there were two groups, we would use di, and if four, tetra.
Step 3. Indicate the position of all of the side groups on the longest carbon chain by numbering the carbon atoms. Remember to use the lowest numbers possible, When there are two or more different alkyl groups, they are placed in alphabetical order irrespective of their numbered position on the chain.
We have numbered the carbon atoms on the molecule so that two of the methyl groups have the lowest possible number, 2. You will notice that the numbers in a name are separated by commas, that the numbers and letters are separated by hyphens and that every side group has a number. So the systematic name of iso-octane is: 2,2,4-trìmethylpentane.
The system we have used to name this compound is recommended by the International Union of Pure and Applied Chemistry (IUPAC) and is accepted by chemists throughout the world. However, if you enter the chemical industry, youm may still hear non-systematic names such as iso-octane.
ALKANES FORM A HOMOLOGOUS SERIES
We have already seen that alkanes can be represented by a general formula, CnH2n+2. This makes them a homologous series. The compounds in a homologous series have similar chemical properties, and each subsequent member differs from the previous one by a CH2 group. They can be represented by a general formula.
As you go through my posts you will encounter other homologous series. Chemists find it useful to classify organic compounds into homologous series because of the similar chemical properties of the members. This means they can be studied as a group, rather than studying each individual compound.
Physical properties of alkanes
The term physical properties refers to those properties of a substance that can be measured or observed without changing the composition or identity of the substance. Physical properties include melting point, boiling point, colour and density. In a homologous series the physical properties gradually change as the number of CH2 groups increases. The steady change in the boiling points of the alkanes is used to separate them when crude oil is passed into a fractionating column.
The effect of branched chains on the boiling point
The occurrence of branching on a carbon chain reduces the boiling point. The more branching there is in an alkane, the lower will be its boiling point compared with its straight-chain structural isomer. Pentane and its isomers clearly demonstrate this.
Branching also affects the volatility of an alkane. Volatility describes the tendency of a liquid to evaporate. Branched chain alkanes are very important in blending petrol because of their volatility, as will be seen later on.
INTERMOLECULAR FORCES IN ALKANES: INDUCED DIPOLE-INDUCED DIPOLE
We have seen two trends in the boiling points of alkanes. Straight-chain alkanes show an increase in boiling point as the number of carbon atoms increases, and branching reduces the boiling point when the number of carbon atoms remains the same.
The particles in a liquid are held close together by forces. The stronger the forces are, the higher is the boiling point of the liquid. The forces that hold alkane molecules in a liquid are weak intermolecular forces, which is why alkanes have relatively low boiling points. A molecule of hydrogen chloride is polar because the centres of negative and positive charge do not coincide. In a polar molecule there is a permanent dipole: one part of the molecule always has a slightly positive charge and another part always has a slightly negative charge. Highly polar molecules can often attract each other quite strongly.
Alkanes are sometimes referred to as non-polar molecules because they have no permanent dipole. So how do intermolecular forces arise and cause the molecules to attract each other? And how is the increase in carbon atoms related to the trend of increasing boiling point?
The electrons in atoms and molecules are in constant motion. Although alkanes have no permanent dipole, the electron charge cloud around their molecules is not always evenly distributed. At any one instant, there may be a temporary dipole when the centres of positive and negative charge do not coincide. This instantaneous dipole induces dipoles in neighbouring molecules. A positive charge at one end of a molecule induces a negative charge in a molecule close to it.
These opposite charges can now attract. The force between them is known as an induced dipole-induced dipole force, or a van der Waals force after the Dutch physicist Johannes van der Waals (1837-1923). In the next instant, the movement of electrons in the molecules leads to a collapse of these temporary dipoles and elsewhere in the substance other instantaneous dipoles form. Averaged over time, alkane molecules do not have a permanent dipole, but billions of instantaneous dipoles are continuously occurring, providing a weak intermolecular force.
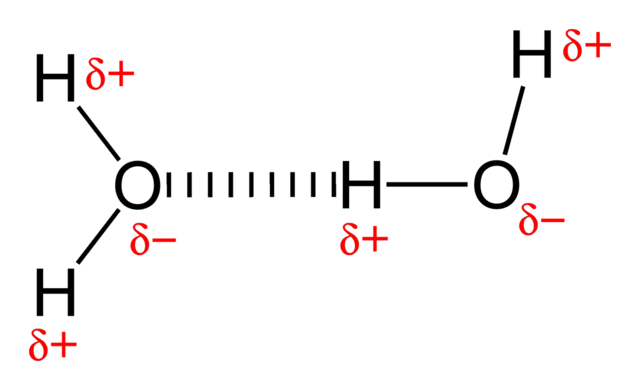
Hydrogen-bonding-in-water-2D. Benjah-bmm27, public domain
The more electrons there are in a molecule, the stronger are the induced dipole-induced dipole forces, because there can be a greater distortion of a charge cloud. As the number of carbon atoms increases in the straight-chain alkanes, so do the number of electrons, which in turn increases the induced dipole-induced dipole forces between the molecules, and so leads to increased boiling points.
Although induced dipole-induced dipole forces are relatively weak, they are extremely important. They explain, for example, why noble gases such as helium can be condensed at 4 K. If there were no forces between helium atoms, they could never form a liquid. Induced dipole-induced dipole forces are present in all substances, but they are most important in those that are non-polar, such as alkanes.
We can use the same model of induced dipole-induced dipole forces to explain why branching in alkanes reduces the boiling point. As we can see that pentane is a straight chain, which allows the molecules to line up beside each other, thereby giving a greater surface area over which these weak forces can act. Branching reduces this surface area and so the intermolecular forces are correspondingly weaker, which leads to lower boiling points.
Getting the volatility right
For petrol to burn in an engine, it must vaporise and be mixed with air. So a crucial property of petrol is high volatility. On extremely cold winter mornings, the petrol must be volatile enough to vaporise and mix with air. If it is not, the engine won’t start. However, on hot summer days, if the petrol is too volatile, it may vaporise in the fuel feed-pipe and so cause a vapour lock that prevents the petrol mixing with air and reaching the engine. Again, the engine won’t start. Another drawback of high volatility is that if the petrol vaporises too readily, too much of it enters the engine and there won’t be enough oxygen for it to burn efficiently.
In the UK, the composition or blend of petrol is changed four times a year, according to the season. So, during the winter, petrol contains more of the alkanes with low boiling points, such as butane and pentane, while in the summer, the proportion of volatile components is reduced and more compounds of low volatility are incorporated.
REACTIONS OF THE ALKANES
Alkanes are quite unreactive because of the nature of the C-C and C-H bonds. First, both are very strong bonds that are difficult to break. Second, both these bonds are non-polar as the electronegativities of carbon and hydrogen are similar. The non-polar nature of the molecules makes them unreactive to polar reagents and ions, and they do not react with acids, bases, metals or oxidising agents such as potassium manganate(VII). It is their very unreactivity that makes them so useful as lubricants and plastics.
The few reactions that alkanes can undergo are of fundamental importance. Two of these reactions – cracking and reforming – are used to produce petrol with desirable properties. Another involves halogens. Cracking also produces alkenes, which are the bedrock of the petrochemical industry. For more about the petrochemical industry, read some of my [previous post on Alkene and the Petrochemical Industry.]
COMBUSTION OF ALKANES
We know that a spark produces a spontaneous reaction between petrol vapour and oxygen. The spark provides the activation energy for this reaction. Alkanes burn in oxygen, releasing a great deal of energy, which is one of the reasons why they are such popular fuels. They all produce carbon dioxide and water when there is enough oxygen for them to burn completely. Butane is used to increase the volatility of petrol. Heptane is another component of petrol. If there is insufficient oxygen, alkanes are not completely oxidised. They produce carbon monoxide, which is very poisonous, and even carbon (soot). This is a real problem when burning petrol in vehicle engines.
The petrol engine
The petrol engine is classed as an internal combustion engine because the fuel burns inside the engine and the products of this combustion drive the engine directly.

Taking a four-stroke cycle on which vehicle engines work, as an instance, the drive to make petrol engines more fuel-efficient centres upon how much the petrol-air mixture can be compressed. In engines of the 1920s, each piston compressed the mixture to a quarter of its original volume. But in today’s engines the petrol-air mixture in each cylinder is compressed to one-tenth of its original volume, thereby extracting much more energy from the combustion of the hydrocarbons.
To be continued…..
REFERENCES
https://www.sciencedirect.com/topics/earth-and-planetary-sciences/smog
https://www.londonair.org.uk/LondonAir/guide/SummerSmog.aspx
https://www.irceline.be/en/documentation/faq/when-do-smog-episodes-arise
https://en.wikipedia.org/wiki/Smog
http://www.chem.uiuc.edu/GenChemReferences/nomenclature_rules.html
https://www.bbc.co.uk/bitesize/guides/z3mpk7h/revision/2
https://www.toppr.com/ask/question/what-effect-does-branching-of-an-alkane-chain-has-on3/
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch02/ch2-4.html
https://www.thebalance.com/volatility-definition-and-types-3305968
https://www.bloomberg.com/opinion/articles/2017-07-10/get-your-volatility-signals-right
https://courses.lumenlearning.com/introchem/chapter/reactions-of-alkanes/
https://chemed.chem.purdue.edu/genchem/topicreview/bp/1organic/reaction.html
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/27%3A_Reactions_of_Organic_Compounds/27.07%3A_Reactions_of_Alkanes
@tipu curate
Upvoted 👌 (Mana: 0/3 - need recharge?)
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!