Hello, great readers. Greetings to the entire steemstem and stem communities. Thanks for the constant support and motivation. Today, I will continue from where I stopped in my previous post on ALKANES, THE CAR ENGINE AND ATMOSPHERIC POLLUTION. I will be discussing on octane number of a petrol blend, blending an unleaded petrol, the greenhouse effect from pollution and some other interesting topics relating to alkane and atmospheric pollution.
OCTANE NUMBER OF A PETROL BLEND
As the petrol-air mixture is compressed in a cylinder, it becomes hot and sometimes ignites without the aid of a spark from the spark plug (auto ignition). When this happens, the fuel does not burn smoothly, but explodes in different parts of the cylinder, which generates a rapid knocking noise. Knocking can damage the cylinder and piston head and, because the petrol-air mixture does not ignite at the right time, it leads to a loss of power and inefficient use of fuel.
It is the job of the petrol blender to produce a knock-free fuel, So blenders use a measure of a blended petrol’s resistance to knocking, call octane number. Remember that 2,2,4-trimethylpentane used to be known as iso-octane, which is why the scale is called octane number or octane rating. Branched-chain alkanes burn more smoothly in an engine than straight-chain alkanes. 2,2,4-Trimethylpentane has a low tendency to a ignite when compressed and is given an octane number of 100. The unbranched chain alkane heptane knocks readily, even under mild compression. It is given an octane number of 0. Mixtures of these two alkanes are used to assign octane numbers to petrol blends. So if, under test conditions, a particular petrol blend knocks at the same compression as a mixture of 90 per cent 2,2,4-trimethylpentane and 10 per cent heptane, the octane number of that petrol blend is 90.

ADDING LEAD COMPOUNDS TO PETROL
The advantage of high-octane fuels is that they can be highly compressed, which gives more power per piston stroke and a more efficient use of fuel. In the 1920s, it was discovered that adding small amounts of a certain lead compound to petrol significantly increased the octane number, a practice that was adopted by all the major petrol suppliers.
However, the use of lead in petrol to raise the octane number is now banned in most countries for two reasons, both of them connected to the pollution of the environment by lead discharged through exhausts. The first is that lead is a poison that accumulates in the bodies of humans and other animals. Evidence suggests that when children breathe in airborne lead, their IQ level is liable to be lowered, and for adults, their ability to concentrate is reduced. Since the advent of unleaded petrol in the UK in 1986, the concentration of airborne lead in the environment has been reduced by 80 per cent. The second reason why leaded petrol is being phased out is that it inhibits the action of catalysts in catalytic converters, which are essential to reduce pollution from vehicle exhausts.
Blending unleaded petrol
If lead is not used to improve the octane number, an alternative way must be found. One solution is to dissolve into the blended petrol small straight-chain alkanes such as butane. The shorter the chain, the less likely is auto-ignition of the mixture. As the alkane chain becomes longer, so the tendency to auto-ignite increases and the octane number decreases. However, too many short-chain alkanes with low boiling points increase the volatility of the petrol too much. This could lead to vapour lock, and it increases evaporation of hydrocarbons from the fuel tank and engine – yet another source of atmospheric pollution.
Using branched-chain alkanes is another way to raise the octane number of petrol. The more branched the chain, the higher its octane number. Branched-chain alkanes are produced at the refinery by cracking and reforming reactions.
A third way is to add aromatic hydrocarbons. Some 40 per cent of the petrol used in the UK contains such additives. One of them is benzene, a known carcinogen (cancer-causing agent), which can make up to 5 per cent by volume of a petrol. Benzene is now being linked to childhood leukaemia, and is thought to be the major cause in some cases. The level of benzene inside a car can be up to ten times higher than outside.
The octane number can also be improved by adding oxygenates. Oxygenates are partly oxidised fuels that already contain oxygen in their molecules. Methanol (CH3OH) has an octane number of 114 and ethanol (C2H5OH) is 111, so they burn smoothly under high compression. Also, because they have an oxygen atom already in the molecule, when they are combusted they require less oxygen in the petrol-air mixture. So petrols that contain oxygenates produce less carbon monoxide when burnt.

POLLUTION FROM VEHICLES AND CATALYTIC CONVERTERS
At the outset of this chapter, I pointed to the problem of vehicle emissions. Now I’m going to look in more detail at the chemistry behind the pollution associated with the burning of hydrocarbon fuels, such as petrol in vehicle engines. Pollution such as: Carbon dioxide increasing the greenhouse effect, carbon monoxide being toxic to all vertebrates. Nitrogen oxides causing respratory problems and acid rain. Unburnt hydrocarbons helping to form photochemical smog and some causing cancer. Suphur oxides causing acid rain, uncontrolled exhaust emissions cause increasing concern, etc.
CARBON DIOXIDE AND THE GREENHOUSE EFFECT
When a hydrocarbon completely combusts, it produces just two products: carbon dioxide and water. Both of these are referred to as greenhouse gases, but what are greenhouse gases and how did they get their name?
The atmosphere of the Earth acts like glass in a greenhouse. For a start, the atmosphere reflects 30 per cent of the Sun’s energy back into space. The remaining 70 per cent, which is mainly visible spectrum light, passes through the atmosphere to strike the Earth. Some of this energy is used by plants for photosynthesis, and some is used to evaporate water from the oceans, lakes and vegetation, but most of it warms the surface of the planet. The warm Earth’s surface in turn emits its own radiation, and this is where certain gases in the atmosphere act like greenhouse glass. The energy radiated by the Earth’s surface is in the long-wave infrared (IR) region of the spectrum. The greenhouse gases absorb this IR radiation, and their molecules become excited.
Three of the most important greenhouse gases are water, carbon dioxide and methane. Quanta of infrared radiation are absorbed by O-H, C=O and C-H bonds, which increase their vibration and thus promote them to higher vibrational energy levels. When they fall back to a lower vibrational energy level, they radiate these quanta of energy in all directions. Some of this energy is transferred through collision of air particles increasing their kinetic energy and warming the atmosphere, but the rest is transmitted back to the Earth’s surface. This is the greenhouse effect. Let’s be reminded that a quantum is an indivisible unit or packet of radiation energy (also called a photon). A molecule can exist only in certain fixed vibrational energy levels. When it absorbs a quantum of energy, it becomes excited and jumps to a higher energy level. In this level, it vibrates more. This is similar to electrons absorbing quanta of energy and moving to higher electron energy levels.
A modern Greenhouse in RHS Wisley. Mark Boyce , CC BY-SA 3.0
Why we need the greenhouse effect in moderation
If carbon dioxide and water were not present in the atmosphere, the Earth would be many degrees cooler. We only have to look at our two closest planetary neighbours to see the effect of greenhouse gases. Venus has an extremely dense atmosphere, 90 times denser at the surface than that of Earth. It is 96 per cent carbon dioxide and keeps the temperature on Venus to a scorching 450 °C. The atmosphere of Mars has about the same proportion of carbon dioxide as that on Venus, but is only 1 per cent as dense as the Earth’s atmosphere. Mars is further from the Sun than Venus so you would expect Mars to be cooler, but it has greater temperature fluctuations and cools during its night to -80 °C because there are not enough carbon dioxide molecules to trap the IR radiation.
The greenhouse effect and climate change
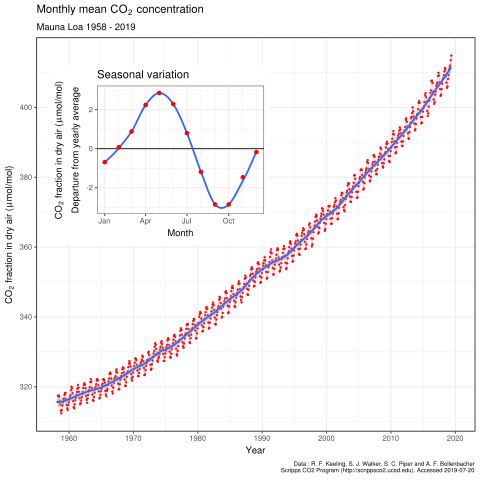
In 2007 there were about 2850 gigatonnes of carbon dioxide in the Earth’s atmosphere, but its concentration in air is small at 0.0383 per cent, or 383 parts per million (ppm) by volume. We know from analysing polar ice cores that the atmospheric concentration of carbon dioxide has varied between 180 ppm and 300 ppm during the last 650 000 years, and at the beginning of the Industrial Revolution, 250 years ago, there were about 280 ppm of carbon dioxide. Most of the increase in carbon dioxide concentration we see today has happened in the past 50 years. This is clearly shown below which is one of the most important graphs in history, produced from results of the Mauna Loa Observatory in Hawaii.
At present, the rate of increase in carbon dioxide is about 2 ppm per year, which means that about 14 Gt is added to the atmosphere (Gt is called a gigatonne which is 109 tonnes). However, more than 28 Gt of carbon dioxide is produced each year, much of it from burning fossil fuels for electricity generation or transport. So, where does half the carbon dioxide go? Some is removed by plants during photosynthesis, and a lot more dissolves in the oceans, lakes and waterways, but there is evidence that oceans are absorbing less CO2 than they did a few years ago. Something that removes carbon in the form of carbon dioxide is known as a sink. So plants and open waters are sinks for carbon. Crude oil is another sink for carbon, and so are the bodies of animals. The amount of time carbon spends in a sink is known as the residence time. Carbon capture and storage is the name given to the removal of waste carbon dioxide. It can be liquefied and injected deep into the oceans. Carbon dioxide can also be stored in geological formations. This is happening now in some of the Norwegian oil fields that have come to the end of their production. It can also be reacted with metal oxides to form stable carbonate minerals.
Recognizing that global climate change could result from increasing concentrations of greenhouse gases in the atmosphere, the World Meteorological Society and the United Nations established the Intergovernmental Panel on Climate Change (IPCC) in 1988. This organisation assesses all the evidence from thousands of scientists researching into climate change, in order to come to conclusions. Its latest report was produced in 2007. On the eve of its publication, the Paris authorities switched off the lights around the city to acknowledge the seriousness of the report’s findings. The report says that it is 90 per cent certain that human activities are causing global warming and climate change. This was important because many scientists in the past have argued that global warming is part of a natural cycle and not the result of human activity. For its work the IPCC was awarded the Nobel Prize, together with former US Vice President Al Gore, who has also done much to publicise the worrying implications of climate change.
The Kyoto Protocol was the first international attempt at curbing greenhouse gas emissions. It was hammered out in 1997 at the city in Japan in that bears its name. It committed industrialised nations to legally binding targets to limit the emissions of six greenhouse gases (most important being carbon dioxide) to 5.2 per cent below their 1990 levels by the period 2008-2012. In 2005 the Kyoto Protocol came into force when sufficient countries had signed up to account for 55 per cent of the 1990 carbon dioxide emissions. The number of countries that have now signed is more than 141, but significantly the USA and Australia refuse to support the agreement. The USA alone previously accounted for almost one-quarter of all carbon dioxide emissions. This country was the biggest emitter of carbon dioxide until it was overtaken by China, which was building two coal-fired power stations each week in 2007. However, it would be unfair for developed countries to point the finger at China. The average Chinese person emits 3.5 tonnes of carbon dioxide per year, compared with the average Briton who emits 10 tonnes or the average US citizen who emits 20 tonnes.
The situation is now considered very urgent and many scientists believe that political action is falling a long way short of what is needed to avert lasting damage to Earth’s climate.
REFERENCES
https://en.wikipedia.org/wiki/Octane_rating
https://www.pei.org/wiki/octane-number
http://www.todayifoundout.com/index.php/2011/11/why-lead-used-to-be-added-to-gasoline/
https://en.wikipedia.org/wiki/Tetraethyllead
https://www.toppr.com/ask/question/why-is-lead-compound-added-to-petrol/
https://en.wikipedia.org/wiki/Gasoline
https://www.sciencedirect.com/science/article/pii/S0960148109000585
https://www.sciencedirect.com/science/article/pii/S0196890402001668
https://en.wikipedia.org/wiki/Catalytic_converter
https://www.explainthatstuff.com/catalyticconverters.html
https://eurekalert.org/pub_releases/2000-08/ACS-Ccfo-1608100.php
https://www.sciencenewsforstudents.org/article/explainer-co2-and-other-greenhouse-gases
https://en.wikipedia.org/wiki/Greenhouse_gas
https://climatekids.nasa.gov/greenhouse-effect/
https://sciencing.com/importance-greenhouse-effect-3535.html
http://theconversation.com/remind-me-again-how-does-climate-change-work-46
https://archive.ipcc.ch/organization/organization_history.shtml


Hi, thanks for the post! I included a link to it in my daily Science and technology digest, and you'll get a 10% share of that post's rewards.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.