Today's post will be centred majorly on spectroscopy and chromatography. So, let's get to start with the discussion on nuclear magnetic resonance spectroscopy.
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
In nuclear magnetic resonance (NMR) spectroscopy, organic chemists have a very powerful technique for determining the detailed structures of compounds. Like infrared spectroscopy, it uses absorption – but in NMR it is the absorption of radio waves by certain nuclei when they are in a strong magnetic field. Spectroscopy such as infrared and NMR involves the interaction of materials with energy from regions of the electromagnetic spectrum. Nuclear magnetic resonance spectroscopy is also called magnetic resonance imaging (MRI), especially when used in medicine. When a new organic compound is discovered, its NMR spectrum is often the first to be looked at because it gives detailed information about the hydrogen nuclei (protons) in the molecule. It can also be used to investigate other nuclei, such as 13C.
Bruker 700 MHz nuclear magnetic resonance (NMR) spectrometer. Mike25, Public Domain
A hydrogen nucleus acts like a tiny bar magnet because it spins, generating a minute magnetic field. When a strong external magnetic field is applied to a compound that contains hydrogen atoms, many of their spinning nuclei (protons) line up so that their magnetic fields are in the same direction as that of the external field (like a group of compass needles, each one pointing north). However, some of the nuclei directly oppose the external magnetic field. (Imagine a compass needle pointing south!) This requires more energy, so these nuclei are at a higher energy level than the rest.
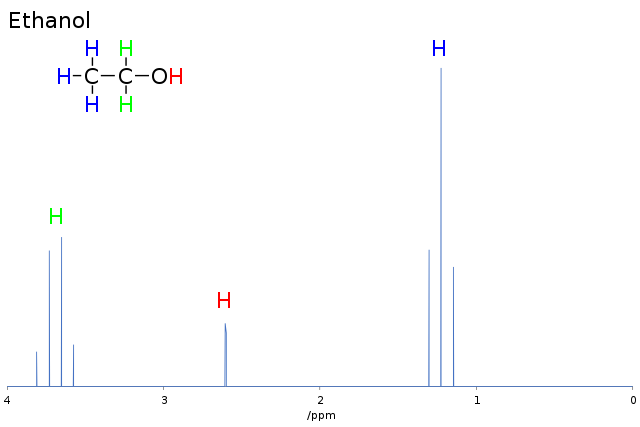
If the compound is now irradiated with a pulse of radio waves of a particular frequency, the lower-energy nuclei absorb these waves and thereby flip to the higher level. This causes a peak in the NMR spectrum. Both the energy needed to promote a hydrogen nucleus (proton) to a higher energy level and the strength of the applied magnetic field experienced by the nucleus depend on the chemical environment of the nucleus. So, hydrogen atoms in different environments in a molecule absorb radio waves in slightly different parts of the spectrum. Take, for example, the ethanol molecule (CH3CH2OH) shown in the figure below.
The hydrogen nuclei are in three different chemical environments. This means that the energy gap between the higher and lower energy levels is different for each of these environments. So, when a pulse of radio waves is applied, more of the lower energy nuclei jump to the higher energy level as they absorb radio waves of a particular frequency. In the case of ethanol, the three peaks in the spectrum correspond to the three different frequencies absorbed by the detector.
A compound called tetramethylsilane, Si(CH3)4, or TMS for short, has all 12 protons (hydrogen nuclei) in the same chemical environment. It therefore gives a very sharp signal. This is used as the standard against which all signal caused by other proton chemical environments are measured, because its protons absorb at a frequency well away from the frequencies of most interest to chemists. TMS is given a value of zero, and the difference between the protons in TMS and the protons in other chemical environments is known as the chemical shift, symbol δ.
The NMR spectrum of ethanol is shown. The area under each peak is proportional to the number of protons in the particular environment. Many NMR spectrometers give these areas as an ‘integration trace’ superimposed on the NMR spectrum. By measuring the height of each step on the integration trace, the ratio of the protons in each environment can be worked out.
USING THE NMR SPECTROMETER
The sample is dissolved in a solvent that does not contain hydrogen atoms, such as CCI4, or a solvent in which hydrogen has been replaced by its isotope deuterium. These solvents are called deuterated solvents, an example of which is CDCl3. The solution is then placed in a strong, magnetic field and a pulse of radio waves passed through it, exciting hydrogen nuclei into their higher spin energy levels. The absorption of radio waves at particular frequencies is detected and recorded. Either the radio frequency or the magnetic field can be varied to give characteristic peaks. Deuterated solvents contain deuterium, D, instead of hydrogen (e.g. CD3COCD3, which is is propanone, CH3COCH3 with the hydrogen atoms exchanged for deuterium). The deuterium nucleus contains a proton and a neutron and does not respond to NMR at the same radio frequencies.
HIGH-RESOLUTION NMR SPECTROSCOPY
The NMR spectra of ethanol and ethylbenzene are at low resolution. High-resolution instruments give more peaks, as shown in the high-resolution NMR spectrum of ethylbenzene. These extra peaks yield more information about the structure of molecules. When different types of proton are next to each other in a molecule, they exert an effect on their respective magnetic fields, known as spin-spin splitting or spin-spin coupling. Look closely at the displayed formula of ethylbenzene. The CH3 protons are next to the CH2 protons, so, they have a direct effect on each other. However, the protons in the benzene ring are not next to any other type of proton.
The CH3 protons experience three different magnetic fields because of the way the CH2 protons spin, while the CH2 protons experience four different magnetic fields because of the way the CH3 protons spin. So, with high-resolution NMR, we can work out the number of nearest-neighbour hydrogen atoms there are in different groups in a molecule. This is made easy to remember by the n + 1 rule: If a proton has n protons as nearest neighbours then its absorption peak will be split into n + 1. This helps us to gain greater insight into the structure of unknown molecules.
Using D2O to identify labile protons
Certain functional groups, such as OH and NH2, rapidly exchange protons with neighbouring molecules. These protons are said to be labile. They usually appear in high-resolution NMR spectra as single peaks, because they do not interact with neighbouring protons. To identify functional groups with labile protons, heavy water (deuterium oxide, D2O) is used. In the case of ethanol, there is an exchange that produces CH3CH2OD, and the peak corresponding to the proton in the OH group disappears.
Imaging the human body with NMR
Nuclear magnetic resonance is becoming increasingly important in medicine, where it is more usually known as magnetic resonance imaging.

The most common nucleus in the body is that of hydrogen, and whole-body scanners investigate tissues by producing images based on the fact that protons in different tissues have different relaxation times. When protons are excited and flipped into higher energy levels, they return to the lower energy stage, emitting energy. The time they take to do this is called the relaxation time. This is detected and the data transformed by computer into an image.
With NMR, doctors can see an image of the soft tissues in the body, which X-rays cannot distinguish. An added advantage is that, unlike X-rays, no side-effects are known, and therefore scans can be safely taken of a patient at regular intervals.
CHROMATOGRAPHY
Chromatography is a method of separating and identifying the components of a mixture. (The name is derived from two Greek words, chroma for colour and graphe for writing.) The technique Was discovered and developed by a Russian botanist, Mikhail Tswett, who first reported it in 1903. He was using powdered calcium carbonate packed into a tube to separate coloured plant pigments called chlorophylls. Before this, chemists mainly used crystallisation techniques to separate and purify substances. Today, chromatography in one form or another is used in nearly all chemical laboratories, not just to separate and purify, but also to analyse substances, most of which are colourless.
All forms of chromatography operate on the same principle. The mixture is introduced into two different phases, of which one remains stationary while the other flows over it. A phase is a homogeneous part of a system that is physically distinct. It is separated from the other parts by a boundary surface. Petrol is a mixture, but it is in one phase, as is a solution of sodium chloride in water. But petrol and water form two physically distinct phases because they do not mix. The phases are known as the stationary phase and the mobile phase, respectively. The components of the mixture distribute themselves differently between the two phases, according to their affinity for each phase. There are two main types of chromatography: partition chromatography and adsorption chromatography.
Partition chromatography
In partition chromatography, the stationary phase is a non-volatile liquid film held on an inert solid surface. The mobile phase is a liquid or gas. The components to be separated distribute themselves between the two phases according to how soluble they are in each.
Adsorption chromatography
In adsorption chromatography, the stationary phase is a solid and the mobile phase is a liquid or gas. The components to be separated are adsorbed on (bonded to) the solid surface of the stationary phase. Those that are only weakly adsorbed travel faster in the mobile phase than those that are strongly adsorbed. Adsorption of reactants by heterogeneous catalysts is a mechanism by which reactions are accelerated.
PAPER CHROMATOGRAPHY
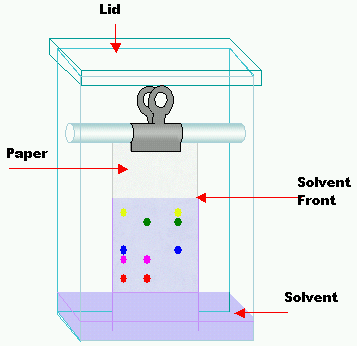
Chromatography using paper is the method you are probably most familiar with. It is an example of partition chromatography. The stationary phase is water adsorbed on the cellulose fibres of the paper. The mobile phase is another liquid solvent.
The sample is spotted along a base-line drawn in pencil on the paper. When the spots are dry, the paper is placed in the solvent, which rises up the paper, taking the components of the sample with it. As they travel up the paper, the components separate according to their different solubilities in the mobile and stationary phases. When the solvent front (the leading edge of the solvent) is almost at the top of the paper, the chromatogram is taken out and dried. If the components are colourless, the chromatogram needs to be developed to make them visible. Sometimes, just heating the chromatogram does this. If not, some other method, such as exposure to ultraviolet light, is used.
Components can be identified by the distances they have travelled up the paper compared with the distance travelled by the solvent front. This is known as the retention factor or retardation factor Rf:
Rf = distance moved by component from base-line/distance moved by solvent front from base-line
To obtain the separated components as samples, their spots are cut out and they are dissolved off in solvents.
Column chromatography
This was the first way in which chromatography was carried out, and it is still the simplest. When Tswett was separating plant pigments, he used a column of calcium carbonate powder as the stationary phase, and a hydrocarbon liquid mixture as the mobile phase. He placed the sample on top of the column, where it made contact with the hydrocarbon mixture, which dissolved the pigments in the sample.
Tswett’s arrangement is still used today. The column is kept topped up with fresh solvent, which washes the pigment down through the inert solid stationary phase (usually aluminium oxide powder or silica gel). The components that are adsorbed most strongly by the stationary phase take the longest time to flow through the column.
When the components are coloured, they can be identified by eye. But if they are colourless, other techniques are used: for example, some components fluoresce in ultraviolet light. Once separation is finished, the solvent is removed by evaporating it off.
Column chromatography in laboratories is usually used to separate minute quantities of mixtures, while in industry it is used in large-scale separation processes that require columns several metres high.
High-performance liquid chromatography (HPLC)
The performance of column chromatography can be improved by using very fine powder as the stationary phase. In this case, gravity alone is not enough to force the mobile liquid phase through the column, and therefore high pressure must be applied. For this reason, this technique is also called high-pressure liquid chromotagraphy, but the abbreviation is the same: HPLC. This is a very efficient process that requires short columns of between 10 and 30 cm in length. Many of the components separated by HPLC absorb ultraviolet light, so it is generally used to identify them.
HPLC has many applications, particularly for the separation and identification of non-volatile substances. Foodstuffs, for example can be checked for additives and contaminants. The detection of steroids in athletes’ body fluids is another example. HPLC can also be linked to a mass spectrometer to identify compounds. This is called HPLC-MS. Fint of all HPLC separates compounds, than the mass spectrometer analyses them. This combination of instruments has been used to identify compounds on the surface of Mars.
REFERENCES
https://www.sciencedirect.com/topics/medicine-and-dentistry/partition-chromatography
https://chromatography.conferenceseries.com/events-list/partition-chromatography
https://byjus.com/chemistry/adsorption-chromatography/
https://www.sciencedirect.com/topics/chemistry/adsorption-chromatography
https://owlcation.com/stem/What-is-Paper-Chromatography-Principle-Uses-experiment-video
https://en.wikipedia.org/wiki/Paper_chromatography
https://www.jove.com/science-education/10217/column-chromatography
https://byjus.com/chemistry/column-chromatography/
https://en.wikipedia.org/wiki/Column_chromatography
https://en.wikipedia.org/wiki/High-performance_liquid_chromatography
http://chem.ch.huji.ac.il/nmr/whatisnmr/whatisnmr.html
https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance
https://en.wikipedia.org/wiki/Spectrometer
https://www.atascientific.com.au/spectrometry/
https://www.chemguide.co.uk/analysis/nmr/highres.html
https://www.nanalysis.com/nmready-blog/2017/11/30/to-d2o-or-not-to-d2o
https://en.wikipedia.org/wiki/Magnetic_resonance_imaging
https://en.wikipedia.org/wiki/Magnetic_resonance_imaging
https://www.sciencedirect.com/book/9780122839801/nmr-in-physiology-and-biomedicine
https://en.wikipedia.org/wiki/Chromatography
https://www.britannica.com/science/chromatography

This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app. This granted you a stronger support from SteemSTEM. Note that including @steemstem in the list of beneficiaries of this post could have yielded an even more important support.