Doctors often need to use X-rays to look inside the intestine and the stomach for cancers, ulcers and blockages.
An image of an X-ray, Pixabay.
However, using X-rays to diagnose
such conditions does pose a problem, because the stomach and the intestine are
transparent to X-rays (they pass through), so no image is detected – unlike
bone, which is opaque to X-rays. To overcome this, doctors give their patients
a barium meal that contains insoluble barium sulphate and water. The stomach
and intestine become coated with the barium sulphate, which is opaque to
X-rays, and so a shadow of these organs is cast on an X-ray photographic film.
Using barium sulphate has one drawback: aqueous barium ions are highly toxic. Even though barium sulphate is said to be insoluble, there is still a small concentration of aqueous barium ions in the stomach, which could lead to poisoning. The equilibrium equation shows the production of barium ions:
BaSO4(s) ⇌ Ba2+(aq) + SO42-(aq)
The concentration of the aqueous barium ions is reduced by the common-ion effect (check below), brought about by magnesium sulphate added to the barium meal. The aqueous sulphate ions from the soluble magnesium sulphate shift the equilibrium to the left to lower the concentration of aqueous barium ions.
Common-ion effect
Since the process involved in the solubility product is a dynamic equilibrium, it is possible to change the position of equilibrium according to [LeChatelier’s principle]. Consider the solubility of barium carbonate. Aqueous barium ions and aqueous carbonate ions are in equilibrium with solid barium carbonate:
BaCO3(s) ⇌ Ba2+(aq) + CO32-(aq)
When extra carbonate ions are added to the equilibrium mixture, the equilibrium shifts to the left to remove the extra carbonate ions. This causes a reduction in the solubility of barium carbonate, the concentration of aqueous barium ions having decreased. This is an example of the common-ion effect. The solubility of barium carbonate is also reduced by the addition of extra aqueous barium ions.
The common-ion effect can be demonstrated quantitatively by means of the solubility product. Consider the solubility of barium chromate(VI) in:
(a) water and (b) 1.0 mol dm-3 potassium chromate(VI). The solubility product of barium chromate is 2 × 10-10 mol2 dm-6 at 298 K.
It is possible to calculate the solubility in a) and b).
(a) Let s be the solubility of barium chromate(VI).
BaCrO4(s) ⇌ Ba2+(aq) + CrO42-(aq)
At equilibrium: s, s
Therefore:
Ksp = [Ba2+(aq)][CrO4²-(aq)]
= s2
This gives: s = 1.4 × 10-5 mol dm-3.
(b) In this calculation, it is necessary to consider dissolving barium chromate(VI) in aqueous potassium chromate(VI):
BaCrO4(s) ⇌ Ba2+(aq) + CrO42-(aq)
At start/mol dm-3: 0, 1.0
At equilibrium/mol dm-3: s, (1.0 + s)
Barium chromate(VI) is sparingly soluble in water. Therefore s is small compared with 1.0, and so (s + 1.0) is approximately equal to 1.0.
Now:
Ksp = [Ba2+(aq)][CrO4²-(aq)]
2 × 10-10 = s(1.0)
This gives: s = 2 × 10-10 mol dm-3.
Note that the solubility of barium chromate(VI) is very much smaller in potassium chromate(VI) than in water alone. This is the common-ion effect in operation.
PRECIPITATION
Precipitation is the formation of an insoluble solid when two solutions react together. For example, when a solution that contains aqueous barium ions is mixed with a solution that contains aqueous carbonate ions, a white precipitate of insoluble barium carbonate is formed:
Ba2+(aq) + CO32-(aq) → BaCO3(s)
Note that this is the opposite process to the dissolving of sparingly soluble barium carbonate in water. This means that the solubility product predicts the conditions for a precipitation reaction. In fact, precipitation occurs when the ionic product for the compound exceeds its solubility product. The ionic product of barium carbonate is the product of the concentration of the aqueous barium ion and of the aqueous carbonate ion.
EXAMPLE
Will a precipitate of barium carbonate (Ksp(BaCO3) = 8.1 × 10-9 mol2 dm-6) be formed from a solution that is both 0.1 mol dm-3 in aqueous barium ion and 1.0 mol dm-3 in aqueous carbonate ion?
ANSWER:
Ionic product (BaCO3) = [Ba2+(aq)][CO32-(aq)]
= 0.1 × 1.0
= 0.1 mol2 dm-6
Ksp(BaCO3) = 8.1 × 10-9 mol2 dm-6
The ionic product is larger than the solubility product, so a precipitate will be formed.
ALUMINIUM AND BORON
So far, the previous post has concentrated on the behaviour of the Group 1 and 2 elements and their compounds. But there is one other metal that can reasonably be called reactive and that is aluminium. The surface of aluminium is covered by an impermeable layer of aluminium oxide that masks the true reactivity of the metal. Once this layer has been removed, the metal resembles magnesium in its reactivity. Boron is also in Group 3 but it is very different to aluminium, because it is a non-metal rather than a reactive metal.
OCCURRENCE AND MANUFACTURE OF ALUMINIUM
Aluminium is estimated to make up 7.5 per cent of the Earth’s crust, most of it as complex aluminates and aluminosilicates in clays, and also as bauxite, which is a form of aluminium oxide and hydroxide. It is not possible commercially to extract aluminium from clays and so bauxite is the major source of all aluminium metal used today.
Electrolytic manufacture of aluminium
The process dates back to 1886, when Charles Hall and Paul Heroult independently discovered a way to extract aluminium from bauxite using electrolysis. The bauxite mineral is mined and then purified to form pure aluminium hydroxide, which is in turn converted into pure aluminium oxide. This is the substance that is electrolysed to form aluminium.
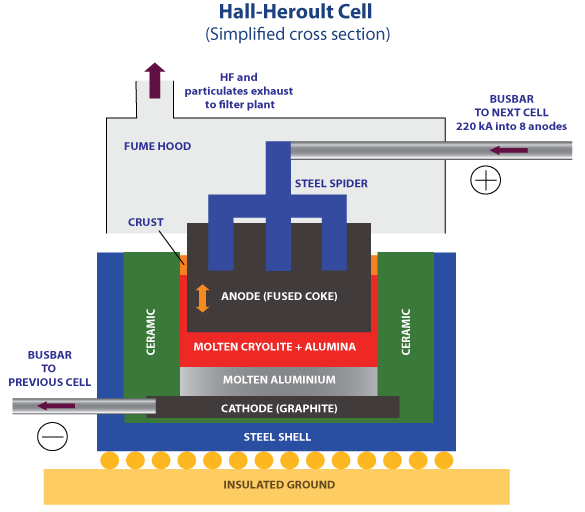
A Hall–Héroult industrial cell. Kashkhan, CC BY-SA 3.0
The aluminium oxide is first dissolved in molten sodium aluminium fluoride (cryolite) and some calcium fluoride, and is then electrolysed. Electrolysis of pure aluminium oxide would be too expensive since very high temperatures are needed to melt it. Introducing the two fluorides allows a much lower temperature to be used. As a result of shortages of sodium aluminium fluoride, some plants use sodium fluoride and aluminium fluoride instead. A carbon (graphite) anode is used. Oxygen is produced by the discharge of oxide ions:
2O2- → O2 + 4e-
The high temperature of production is a nuisance, because the oxygen reacts with the carbon anode to form carbon dioxide. So the carbon anode is continually being replaced. The carbon lining of the cell acts as the cathode, and aluminium is formed by the discharge of aluminium ions. The temperature in the cell is above the melting point of aluminium, so molten aluminium is formed. This sinks to the bottom of the cell, where it is run off and allowed to cool. The aluminium obtained is exceptionally pure, being at least 99.9 per cent aluminium.
Applications of aluminium
As soon as it was discovered, aluminium found many applications associated with its low density and its resistance to corrosion. More recent applications include foil (often incorrectly called tin foil) for use in the home and in packaging, double-glazed window frames and now car engines and bodies.

Aluminium can. Marcos André, CC BY 2.0
CHEMICAL PROPERTIES OF ALUMINIUM
The electronic configuration of aluminium suggests that an aluminium atom should lose three electrons from an atom to form an aluminium ion. The loss of three electrons is always energetically more difficult than the loss of one or two electrons, and the cation formed will always be small and have a large charge density. This means that the small and highly charged cation is able to attract back the electrons it has lost in forming an ionic bond. An aluminium ion is said to polarise the anions, and so many aluminium compounds have a covalent character. In aqueous solution, six water molecules surround an aluminium ion to form a hydrated ion, [Al(H2O)6]3+(aq). For simplicity, this is often written as Al3+(aq).
Reaction with air and oxygen
As already noted, aluminium forms an oxide layer on its surface which protects the rest of the metal from further corrosion. When heated in air or oxygen, aluminium powder burns to form aluminium oxide, a white ionic solid. If the oxide layer is removed, for example by wiping the surface with mercury, then aluminium will react with water to form hydrogen.
Reaction with acids
Aluminium powder reacts with dilute sulfuric acid and dilute hydrochloric acid to give the corresponding aqueous aluminium salts. These reactions are initially very slow and need heat to accelerate the reaction rate. But, once started, they become quite violent. The initial slow rate of reaction arises from the time it takes for the acid to react with the protective oxide layer before it exposes the active metal. Surprisingly, aluminium does not react with pure nitric acid. It is therefore used as the lining of vessels to transport this acid.
Reaction with alkalis
Aluminium is unusual in that it reacts with both acids and alkalis. This property makes aluminium an amphoteric metal. Aluminium powder reacts vigorously – after a slow start – with warm aqueous sodium hydroxide to form hydrogen and aqueous sodium aluminate.
Reaction with halogens
When one of the halogens is passed over heated aluminium, the corresponding halide is formed by direct combination. The halides formed have a large degree of covalent character:
2Al(s) + 3X2 (g) → Al2X6(s)
Where X = a halogen atom.
COMPOUNDS OF ALUMINIUM
Most aluminium compounds have some covalent character, but when dissolved in water or as hydrated crystals, the compounds are ionic, and contain the hydrated aluminium ion.
Aluminium oxide and aluminium hydroxide
Both these are white ionic amphoteric solids. With acids, they form the hydrated aluminium ion [Al(H2O)6]3+; with alkalis they form the aluminate ion [Al(OH)4]‑ in which the aluminium atom forms part of the anion.
Aluminium oxide is used as an abrasive and as a dehydrating agent, particularly in the formation of alkenes from alcohols. Aluminium hydroxide is used in the dyeing industry, since it can adsorb coloured materials onto its surface. This property is also used to remove particulate matter from water during its purification. In this application, a solution of aluminium sulphate is added to the water and forms aluminium hydroxide, which, as it settles, collects particulate matter and removes it from the water. The amount of aluminium sulphate used in this process has to be carefully controlled to prevent excess aluminium ions reaching domestic water supplies, since aluminium ions may be linked to Alzheimer’s disease.
Solutions of aluminium salts
Aqueous solutions of aluminium salts are highly acidic, so much so that they can release carbon dioxide from sodium hydrogencarbonate. This acidity is caused by hydrolysis and involves one of the water molecules in the aqueous aluminium cation being polarised to form an aqueous proton. All aqueous solutions of aluminium salts react with aqueous hydroxide ions to give first a white precipitate of hydrated aluminium hydroxide and then, with excess hydroxide ions, a colourless solution of the aluminate ion. This reaction involves deprotonation, in which each reaction step removes one more proton from the aluminium species.
IONIC SOLVENTS
One of the typical properties of an ionic salt is that it has a high melting point. Often an ionic salt has to be heated to above 700°C before it will melt and produce an ionic liquid. However recent research has developed ionic liquids at room temperature. All that needs to be done is to mix together and warm gently certain powdered organic salts with anhydrous aluminium chloride; the result is a clear, colourless, ‘ionic liquid’. The ionic liquids contain an organic cation and a tetrachloroaluminate anion, AICI4-. These ionic liquids may well be a possible alternative to using standard organic solvents to dissolve substances. Many industrial processes use volatile organic compounds, known as VOCS, as solvents. These solvents are often toxic and flammable; they vaporise easily and are greenhouse gases and/or ozone-depleting pollutants.
The new ionic liquids are non-volatile and are being used to develop new processes that use clean-technology solvents. Uses in chemical syntheses, catalysis and metal finishing are all being developed. Ionic liquids may reduce atmospheric pollution, but chemists have to research any possible environmental concern about the disposal of large quantities of these ionic liquids.
BORON
Even though boron is in Group 3, it is a non-metal and it forms compounds by sharing electrons rather than by losing electrons. Boron trichloride, BCl3, is a typical simple molecular chloride that hydrolyses in water; this is the typical behaviour of a non-metal chloride.
Forms of boron nitride
When chemists discovered the two forms of boron nitride they noticed how similar the structures were to graphite and diamond.
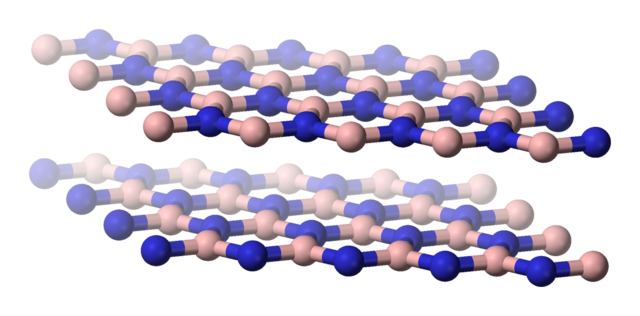
Boron-nitride-(hexagonal)-side-3D-balls. Benjah-bmm27, Public Domain
As a result, the chemists realised that they would have very similar physical properties as well. Boron nitride can be used as a lubricant in the same way as graphite. The layers of boron-nitrogen hexagons can slide over one another very easily, since they are held in place by weak van der Waals forces. Boron nitride remains a lubricant even at high temperatures, unlike graphite. Another advantage over graphite is that it is much less likely to react with oxygen, even at high temperature. It is used in areas of metal extrusion, plastic extrusion and the hot pressing of metals.
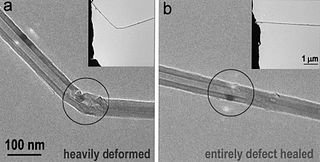
Boron nitride can also be used as an abrasive and in glass cutting, in the same way as diamond. One disadvantage of boron trinitride is that is more brittle than diamond. Knowing that carbon forms fullerenes and nanotubes, chemists developed boron trinitride nanotubes also as shown in the figure above.
CONCLUSION
Going through all the series of my post on reactive metals starting with; Carbon Dioxide Sink and The Reactive Metals, Compounds of Alkali Metals and the Thermal Decomposition of Ionic Salts, Solubility of Ionic Salts, and this final post, you would have learnt that:
- Group 1 metals are highly reactive metals. Each atom loses one electron when it reacts. In compounds, the oxidation state of Group 1 metals is always +1.
- Group 2 elements are less reactive than the group 1 element in the same period. In compounds, the Group 2 elements always have an oxidation number of +2.
- The ease with which an atom loses electrons increases with increasing atomic (proton) number in both group 1 and Group 2.
- The oxides and hydroxides of Group 1 and group 2 elements are basic and when they dissolve in water, they form strongly alkaline solutions.
- The nitrates of Group 1 (except lithium nitrate) thermally decompose to form the corresponding nitrites and oxygen, whereas the nitrates of group 2 thermally decompose to give nitrogen dioxide, oxygen and the corresponding metal oxides. The thermal stability of the nitrates increases with increasing atomic (proton) number of the metal.
- The carbonates of Group 1 (other than lithium carbonate) do not decompose at Bunsen burner temperatures, but most carbonates of Group 2 do to give carbon dioxide and the metal oxides. The thermal stability of the carbonates increases with increasing atomic (proton) number of the metal.
- Lattice enthalpy is the enthalpy change when 1 mole of an ionic lattice is formed from its constituent ions in the gas phase. Lattice enthalpy depends on the charge densities of the ions involved.
- The solubility of the Group 2 sulphates. MSO4 and the Group 2 carbonates, MCO3 decreases with increasing atomic (proton) number of M. This trend is explained by the decrease in the magnitude of the enthalpy change of hydration of M2+.
- The solubility of the Group 2 hydroxides, M(OH)2, increases with increasing atomic (proton) number of M. This trend is explained by the decrease in magnitude of the lattice enthalpy of M(OH)2.
- The thermal decomposition of nitrates and carbonates is determined by the ability of the cation present to polarise the large carbonate and nitrate ions.
- Aluminium is manufactured by the electrolytic decomposition of molten aluminium oxide dissolved in sodium aluminium fluoride.
- Aqueous aluminium salts are acidic because of the polarisation of water molecules by the very small and highly charged aluminium ion.
- The solubility product can be used to explain the common-ion effect and precipitation.
Thanks a bunch for reading.
REFERENCES
https://en.wikipedia.org/wiki/Upper_gastrointestinal_series
https://www.mydr.com.au/tests-investigations/barium-swallow-and-barium-meal-tests
https://www.news-medical.net/health/What-is-a-Barium-Meal.aspx
https://en.wikipedia.org/wiki/Common-ion_effect
https://en.wikipedia.org/wiki/Precipitation_(chemistry)
https://www.chemicool.com/definition/precipitate.html
https://www.britannica.com/science/aluminum
https://en.wikipedia.org/wiki/Hall%E2%80%93H%C3%A9roult_process
https://scienceaid.net/chemistry/applied/aluminium.html
https://www.bbc.co.uk/bitesize/guides/zhk6pbk/revision/4
https://www.chemguide.co.uk/inorganic/extraction/aluminium.html
https://www.austenknapman.co.uk/blog/commercial-metal-use/5-most-common-applications-of-aluminium/
https://www.rsc.org/periodic-table/element/13/aluminium
https://en.wikipedia.org/wiki/Aluminium
https://www.lenntech.com/periodic/elements/al.htm
https://www.alunorf.de/alunorf/alunorf.nsf/id/FC78CEA3DEA1C128C12578F400465232
https://en.wikipedia.org/wiki/Compounds_of_aluminium
This post has been curated by Steemitas Club. Congratulations @empressteemah and thank you for sharing this original work.
If you are not a member of @steemitasclub, we cordially invite you to join our discord server (https://discord.gg/dYMsF7a) to promote your publications.
Thanks, @steemitasclub. I really appreciate.
Hello,
Your post has been manually curated by a @stem.curate curator, @alby2.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
If you like what we are doing, please show your support as well by following our Steem Auto curation trail.
Please join us on discord.
Thanks, @stem.curate. I really appreciate. Thanks also, @alby2
This post has been voted on by the SteemSTEM curation team
and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
I shared this post from my Twitter account: