Some people think of carbon dioxide as just the greenhouse gas that causes global warming problems, although of course carbon dioxide is essential for life as well. From earliest times on Earth and continuously since then, vast amounts of carbon dioxide from the atmosphere have been locked up in rocks of the Earth’s crust as carbonates.
Several different carbonates occur in rocks, but by far the most abundant is that of the highly reactive metal calcium, calcium carbonate, which we see in the enormous chalk cliffs and widespread chalklands in the UK, and also in limestone deposits and coral reefs around the world.
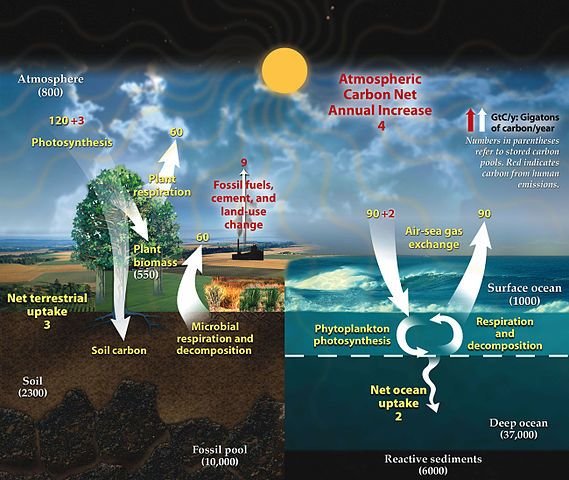
Chalk, limestone and coral reefs exist only because calcium carbonate is an insoluble material, while other calcium salts are soluble. The calcium carbonate comes from the activity of marine animals and microscopic organisms that synthesize the carbonate as part of their life processes, but they can only do this by using calcium salts that are soluble in the waters around them. The calcium carbonate forms their shells, bones or coral protective structures, and as the organisms die and reach the ocean floor, these deposits feed into the rock-forming cycle.
Calcium carbonate formed at the bottom of seas and oceans is therefore a ‘sink’ for carbon dioxide. It has been suggested that, as atmospheric carbon dioxide increases, this will to some extent be offset by an increase in the carbon dioxide content of the oceans and, in turn, by an increase in the conversion of it into calcium carbonate by marine creatures.
REACTIVE METALS
The classification of elements into metals and non-metals is based upon the physical and chemical properties of the elements and their compounds. To most people, metals are hard, strong and shiny and are good thermal and electrical conductors. These properties describe the metals we are familiar with outside the chemistry laboratory, such as iron, lead, silver, gold, zinc, copper and nickel.
Other elements are also classified as metals, but they are less recognizable as such, in that they are soft and have a low melting point. It is only when their chemical properties are described that they are clearly seen to be metals.
These include lithium, sodium, potassium, calcium and barium, which are found in the s block of the Periodic Table and are often collectively called the reactive metals.
Aluminium, in Group 3, is sometimes also referred to as a reactive metal. So aluminium is included in this chapter.
CHEMICAL PROPERTIES OF METALS
The main chemical property of a metal atom is its ability to lose one, two or sometimes three electrons to form a positive ion. When an atom loses electrons, it often attains a stable, noble-gas electron configuration. It is this single property that can be used to explain the reactivity of metals. Atoms of reactive metals easily lose electrons; atoms of unreactive metals lose them with difficulty.

Caesium metal in a glass ampoule. Dnn87, CC BY-SA 3.0
The ability of a metal atom to lose electrons to form a positive ion explains why metals are reducing agents. It also explains why metals form ionic compounds with non-metals; and why metals do not normally form compounds with other metals. OIL RIG: Oxidation is Loss of electrons and Reduction is Gain of electrons. So metals are reducing agents because they give away electrons easily to an oxidizing agent. The most reactive of all metals are those in the s block of the Periodic Table, since they have an electron configuration that contains only 1 or 2 electrons more than the nearest noble gas. This means that atoms of these elements lose electrons easily to form cations. Lithium has the strongest metallic bonding and caesium the weakest metallic bonding of the alkali metals, because the lithium ions have the largest charge density and so the strongest attraction to the sea of delocalized electrons. This is why lithium has the highest boiling point and melting point and caesium the lowest.
GROUP 1: THE ALKALI METALS
The metals in Group 1 are collectively known as the alkali metals. There are six of them: lithium, sodium, potassium, rubidium, caesium and francium. They are the most reactive metals in their appropriate period of the Periodic Table.
These six elements are all remarkably similar in terms of both their chemical and physical properties. Being in the same group, we would expect the elements to have similar chemical properties. However, that many of their physical properties are also similar and with, in some cases, an observable and predictable trend is unusual for most groups within the Periodic Table. For example, the melting and boiling points decrease with increasing atomic (proton) number.
It is interesting that the melting points of these elements are very low for metals, which makes them easy to melt. Advantage is taken of this property in some nuclear reactors, in which liquid sodium is used as the primary coolant because it also has a relatively high specific heat capacity and can be pumped easily through the pipes of the cooling system.

Potassium metal. Wikipedia, CC BY 1.0
OCCURRENCE AND EXTRACTION
The physical properties of the alkali metals, coupled with their high reactivity limit the number of large-scale applications. Nevertheless, there is significant demand for lithium, sodium and potassium.
The alkali metals are so reactive that they occur naturally only as compounds. It is impossible to reduce alkali metal compounds using a chemical process such as heating with carbon. The only successful method of reduction involves electrolytic decomposition. Francium, one of the last of the alkali metals to be discovered, is highly radioactive and, although its chemistry is easily predicted, it has not been fully determined experimentally. This is hardly surprising, since it is estimated that only 15 grams of francium occur in the whole of the Earth’s crust.
MANUFACTURE OF SODIUM
Sodium is manufactured by the electrolysis of molten sodium chloride. The melting point of sodium chloride is 801 °C and so calcium chloride is added to lower the melting point to about 600 °C. The overall reaction is represented by the equation:
2NaCl(I) → 2Na(l) +Cl2(g)
Electrolysis is the only practical way to achieve this decomposition. Sodium ions are reduced at the cathode and chloride ions are oxidized at the anode. Since sodium is a highly reactive metal, it is important to ensure that the sodium and chlorine formed are not allowed to recombine. They must be produced in different parts of the electrolytic cell. In the Down’s cell used to electrolyse molten sodium chloride, a fine screen prevents the chlorine from diffusing to the cathode. All the alkali metals are costly to make because of the amount of electrical energy needed.
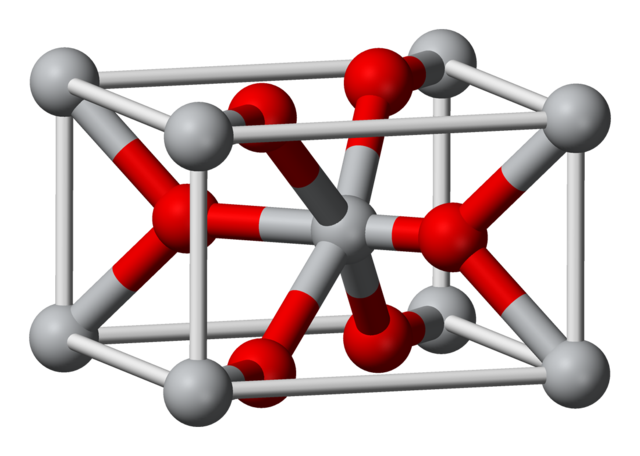
Visual representation of magnesium doped tin oxide structure used in Downs cell. Public Domain
REACTION OF THE ALKALI METALS
The alkali metals are good reducing agents because their atoms can easily transfer the outer electrons to non-metal atoms. Once electrons are lost, the resulting positive ions have noble gas electron configurations. For example, the sodium atom (1s2 2s2 2p6 3s1) loses an electron to form the sodium ion (1s2 2s2 2p6) with the same electron configuration as argon. This means that in all compounds the elements of Group 1 have an oxidation state of +1.
FIRST IONIZATION ENERGY AND ELECTRODE POTENTIALS
Lithium has the highest of the first ionization energy values, so it is expected to be the least reactive of the alkali metals. The first ionization energy refers to the loss of an electron from a gaseous atom, which does not represent the situation for most reactions. As the atomic (proton) number increases down the group, the number of inner-shell shielding electrons increases and the atomic radius increases. The outer electron is therefore attracted less strongly to the nucleus and so less energy is needed to remove it from the atom. This means that the first ionization energy decreases within a group as the atomic (proton) number increases. A more appropriate way to describe the ease of electron loss from an alkali metal atom is to refer to its oxidation potential, Eoxid, since this refers to the reaction of a metal atom to form an aqueous metal ion. For example, the oxidation potential for lithium refers to the following half-equation:
Li(s) → Li+ (aq) + e-
This is precisely the reaction that occurs when lithium metal reacts with water or dilute acid. The values of the oxidation potentials show that all the elements in group 1 are highly reactive in that they can lose electrons easily.
Reaction with water
The alkali metals are so-called because of their reaction with water. All the metals react vigorously with water to form hydrogen and an alkaline solution. Take lithium as an example. During the reaction, the metal reduces the water by losing electrons to form lithium cations and hydrogen. Lithium reacts with water to form aqueous lithium hydroxide and hydrogen:
2Li(s) + 2H2O(l) → 2LiOH(aq) + H2(g)
The ionic equation more clearly describes why an alkali is formed, since aqueous hydroxide ions are formed:
2Li(s) + 2H2O → 2Li (aq) + 2OH‑ (aq) + H2(g)
The reactivity of the alkali metals increases with increasing atomic number, so that the reaction of potassium with water is often accompanied by a lilac flame as the hydrogen produced burns. Sodium reacts with water to form aqueous sodium hydroxide and hydrogen. A yellow flame occurs when the hydrogen produced burns. Notice that sufficient energy is transferred to the surroundings to melt the sodium into a sphere. Potassium is more reactive than sodium or lithium. It reacts violently with water, to produce hydrogen and aqueous potassium hydroxide. The reaction is exothermic and the energy transferred is sufficient to melt the potassium and to ignite the hydrogen formed.
Reactions with acids
The alkali metals react explosively with dilute acids, such as hydrochloric acid and sulfuric acid. They will even displace hydrogen from very weak acids such as alcohols, to form compounds called alkoxides. (The reactions of alkali metals with
Reaction with air
Unlike most metals, the alkali metals are very soft and easy to cut with a knife. The surface obtained after cutting is shiny, but almost immediately tarnishes by reaction with moisture and/or oxygen from the air. All the alkali metals react with air to form a complex mixture of compounds, including the corresponding carbonate.
Reaction of alkali metals with oxygen
All alkali metals burn when heated in oxygen, to produce oxides. The combustion is always accompanied by a coloured flame characteristic of the element. Lithium burns with a red flame, sodium with a yellow flame and potassium with a lilac flame. The colour of a flame is a result of electron excitation and the consequent release of energy as the electron falls back to a lower energy level. The oxides formed are not always the predicted M2O, containing the M+ and O2- ions. Peroxides that contain the O22- ion and superoxides that contain the ion O2- are formed with the more reactive alkali metals. The stability of the peroxides and the superoxides increases with the size of the cation. So, caesium oxide forms CsO2 when burnt in excess oxygen, whereas lithium forms Li2O.

A reaction of 3 pounds (≈ 1.4 kg) of sodium with water. Ajhalls, Public Domain
Potassium superoxide
Superoxides are solids. They are very powerful oxidizing agents, and can oxidise water to form oxygen. This is the reaction in some types of breathing mask used in mine rescue, where potassium superoxide is the source of the emergency oxygen supply to the wearer. Moisture reacts with the superoxide to provide this oxygen:
4KO2(s)+ 2H2O(l) → 4KOH(s) + 3O2(g)
Note that 4 moles of potassium superoxide provide 3 moles of oxygen. This means that one gram of potassium superoxide can provide approximately 250 cm3 of oxygen at room temperature and atmospheric pressure. The attraction of this reaction is that, as the superoxide is used up, it makes potassium hydroxide, which removes carbon dioxide. This prevents the user from breathing in large quantities of carbon dioxide:
2KOH(s) + CO2(g) → K2CO3(s) + H2O(l)
The overall effect is the removal of carbon dioxide and formation of oxygen. Potassium superoxide can also be used in submarines to provide oxygen, while at the same time it prevents the build-up of dangerous levels of carbon dioxide.
I will like to stop here for now. In my next post, I will explain more on the compounds of alkali metals, alkali metal oxide, hydroxides, oxy-salts, etc.
Thanks for reading.
REFERENCES
https://www.eurekalert.org/pub_releases/2019-03/kift-lcd031319.php
https://www.livescience.com/32354-what-is-a-carbon-sink.html
https://en.wikipedia.org/wiki/Carbon_sink
https://www.sciencedaily.com/terms/carbon_dioxide_sink.htm
https://www.bbc.co.uk/bitesize/guides/z3xn82p/revision/1
https://en.wikipedia.org/wiki/Sodium
https://en.wikipedia.org/wiki/Castner_process
https://www.britannica.com/science/alkali-metal
https://opentextbc.ca/chemistry/chapter/18-1-periodicity/
https://www.bbc.co.uk/bitesize/guides/z3xn82p/revision/2
https://www.bbc.co.uk/bitesize/guides/zqwmxnb/revision/2
https://www.britannica.com/science/sodium/Chemical-properties
https://www.chemguide.co.uk/inorganic/group1/reacto2.html
https://www.bbc.co.uk/bitesize/guides/zdykw6f/revision/3
Thank you for your patience in explaining this issue. I can see that you resisted the easy path, which would have been to speak only to people with your level of expertise. I read the first part carefully and even went off to explore the possible complications of using sodium in nuclear reactors--I remembered vaguely the danger of explosion but only vaguely. (Found this: https://www.scientificamerican.com/article/can-sodium-save-nuclear-power/)
The point of my comment is this: even if some of the article was complicated for me, reading it was a worthwhile exercise. I will read it again at bedtime on my iPad. I'm particularly fascinated by the idea of sea life serving as living carbon sink.
Thank you for this interesting read. I'm sending it out on Twitter in the hope that others will enjoy it also.
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
If you like what we are doing, please show your support as well by following our Steem Auto curation trail.
Please join us on discord.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.