Hi guys! Today I want to continue in series about novel method termed optogenetics that can control the activity of the cells with light. It is usually used in neurobiology, but there is also another application that is probably even more peculiar. Because you can even control the activity of genes using this method.
Intrigued? Read on!

Optogenetics 101
I know I do this all the time. Revision time. Optogenetics represent a technique that combines optics and genetics with the aim to control the activity of the cells. This is due to the light-sensitive proteins that are being expressed in the cells. Mostly used cells are neurons and most frequently used light sensitive proteins are termed opsins that are also ion channels. Opening or closing these channels results in change of membrane potential of neurons and thus activating or deactivating them. If you want to know more, then there is list of links at the bottom of the articles that will redirect you to my previous posts about optogenetics (I write there about all of this in more detail).
Different type of light-sensitive proteins
The basic principle of optogenetic use in neuronal signalling and intracellular signalling is the same. You shine light onto the cells and due to light sensitive proteins present the cell does something. But in the case of optogenetic control of intracellular signalling different light-sensitive proteins are used. These are usually not ion channels, but molecules that change their conformation after light stimulation. Does it make sense? If not, don’t worry- I am going to explain everything!
Let’s use Phytochrome B (PhyB) as a demonstration of how it works. It is a protein from plant model organism Arabidopsis thaliana that is necessary for correct growth of this plant. PhyB after being fused with its chromophore PCB gains the ability to change its conformation after stimulation with red light (around 650 nm). After this conformational change the PhyB-PCB complex rapidly binds to phytochrome interacting factor (PIF). This process is reversible and after illumination with infrared light (around 750 nm), thus PhyB returns to its original conformation and dissociates from PIF. This photoactivation is depicted bellow.
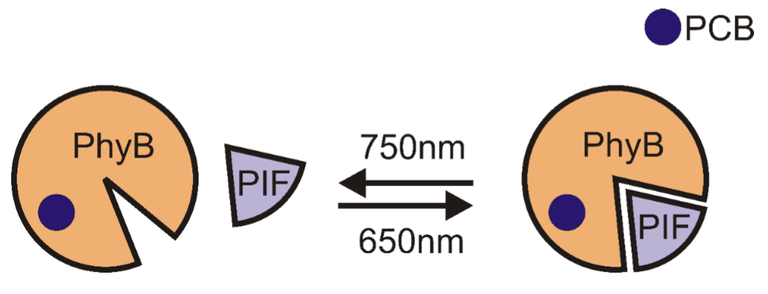
Putting it into action
Now that we know how this might work, lets try to turn it into something beneficial. First a demonstration. In cells there are certain proteins that could be termed anchoring proteins, or just anchors. These proteins have the ability to bind to specific area – such as plasmatic membrane, mitochondrial membrane, etc. Then we have another class of proteins that are termed fluorescent proteins. These proteins exhibit fluorescence when illuminated with light with specific frequency. What can you do with these components, PhyB and PIF? Well you can create fusion proteins – meaning that they are composed of different protein subunits. In this case you can fuse PhyB with anchor and fluorescent protein (such as mCherry). The anchor will be targeted to plasmatic membrane of the cell. Then you can fuse PIF with another fluorescent protein (such as mCitrine). And what you can do is that you can transport PIF to plasmatic membrane. How? Using light with wavelength of 650 nm you change the conformation of PhyB so it will bind to PIF. And because of PhyB being anchored to plasmatic membrane, PIF will happen to be there as well. Subsequent stimulation with infrared light will result in dissociation of PIF from PhyB. You can see the schematics of such experiment bellow. This can be all visualized thanks to the use of fluorescent proteins fused with PIF and PhyB. What does it tell us? That if we fuse PIF and PhyB with different proteins we can transport these proteins of interest whenever we want – and in rapid and reversible manner.
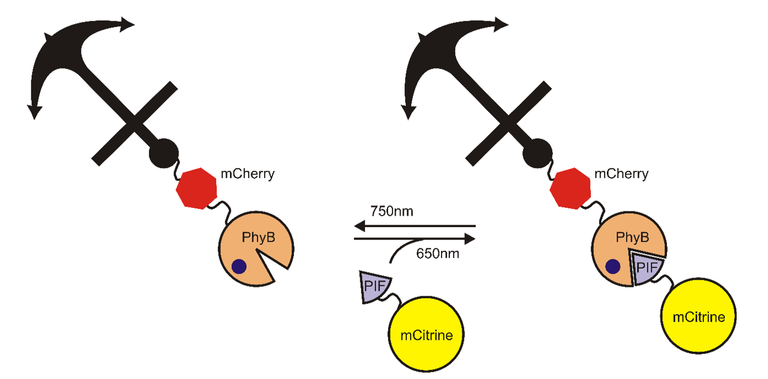
Activating gene transcription
Yang et al. (2013) used this exact strategy to modify the expression of GAL-responsive genes in budding yeast Saccharomyces cerevisiae. Galactose signalling is actually well studied, but this was the first time using optogenetics to modify it. More about this system. Gal80 is the repressor of transcription of GAL-responsive factors. Transcription means that you activate certain gene or genes and thus produce some protein/proteins. GAL-responsive factors are proteins that are being transcribed when galactose is present in cells. When there is no galactose present Gal80 rapidly shuffles between nucleus and cytoplasm and inhibits the transcription of GAL-responsive genes. But, when galactose enters the cell, then Gal80 stays in the cytoplasm and GAL-responsive genes are activated. Take-home message: Absence of Gal80 in the nucleus results in transcription of GAL-responsive genes. This whole process is depicted bellow.
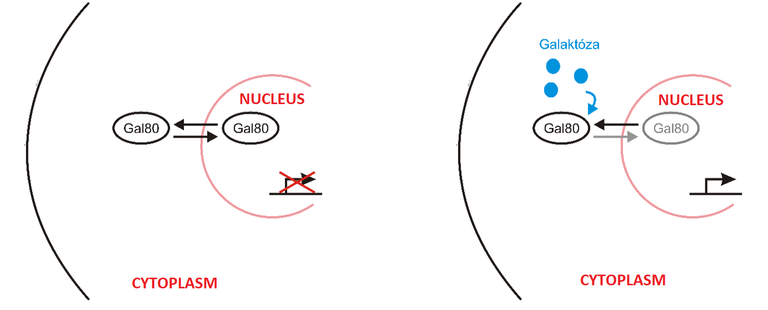
What Yang and his team did was this. They anchored PhyB to the plasmatic membrane and fused PIF with Gal80. And when they illuminated the cells with red light, PhyB changed its conformation and PIF attached to it. Thus, Gal80 was not present in nucleus and that initiated the activation of GAL-responsive factors, even though there was no galactose present in the cell!
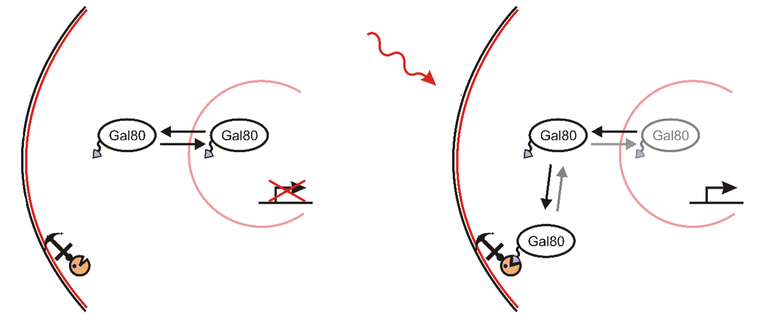
Using this method, you can do plenty of things, there are different proteins such as PhyB that use different mechanisms of action and there are also different anchoring proteins and stuff like that. You can make cells transport their whole organelles somewhere or even make them kill themselves (apoptosis)!
Concluding remarks
Today I introduced you to something even more novel that classical use of optogenetics. There are not that many articles and research teams that use this approach, but I do believe that there is very promising potential.
Hopefully you did enjoy reading this article and learned something new as well. Next time I will probably write more about intracellular activation using optogenetics (if you happen to like it :D ).
Have a great day!
Previous parts of Optogenetics series:
Part 1 – Introduction
Part 2 – History of Optogenetics
Part 3 – Opsins – light sensitive proteins
Part 4 – Gene expression and delivery in Optogenetics
Part 5 – Light stimulation and delivery in Optogenetics
Part 6 – Readout of results in Optogenetics
Part 7 – My own research experience of using Optogenetics
References:
Unsourced images are my own creations.
Being A SteemStem Member
I used channelrhodpsin when I figured out a novel way to reprogram/transdifferentiate human skin cells directly into skeletal muscle. I would shine blue light on the cells, causing the channelrhodopsin to open and depolarize the cell, causing the newly made muscle cell to contract in the dish. The functional readout allowed me to compare efficiencies across multiple conditions to figure out the best one! :)
Wow! It is great to have someone that actually uses the method here. Sounds extremly interesting. You should write an article about it!
Yet another great article from @jepper . Btw I am seeing high quality bio articles via steemSTEM. Kudos to the community!
Should I write similar thing as above? I dont write anything about my own images in my articles. :|
Thank you, I am really glad you liked it! :) To your question - I think it does not matter, I just wanted to state that I did not copy them or something. And I am also a little bit proud about them, so, I wanted to say to everyone that I was able to make them, haha :D
great work and im glad for you,but i cant undearstand how some posts get all that upvotes, best one i had 0.04 maybe, we started we need encouragments from u guys dont be selfish, again im glad for u
I am glad you liked it. Hmm, I actually do not know whether there is a formula on getting upvotes and what I do know is already mentioned in the article, haha. Well, I checked some of your articles (btw, you are huge! :O ) and maybe if you tried doing them a bit longer? Or maybe try to create an article about muscles, how they work or so (add proper references and pictures), then add steemstem tag and maybe you will get more upvotes. I generally write only about science and science-related stuff, so this is probably the only advice I have. :)
thats really kind from u, i ll follow ur advice never too late,there are manythings to learn frm u,i follow u mate