Aeration, one of the simplest forms of oxygen transfer.
Hello to all the members of the great community #steemstem and other enthusiasm of science today I want to start with a series of topics that consist in explaining the transfer of gases and their importance.
So let's begin.
Aeration.
Simply defined, water aeration is essentially the transfer of oxygen from the air to water. Water surfaces require oxygen to fulfill their biological cycle. In large areas of water, this happens naturally as a result of the action of the waves.
Through this process, the water is put in direct contact with the air to modify the concentrations of volatile substances that it possesses.

Source
Gas transfer.
It is a phenomenon that occurs when the molecules of a gas are exchanged between a liquid and a gas at a gas-liquid interface causing the increase in the concentration of gas or gases in the liquid phase as long as this stage is not saturated with the gas at certain given conditions, such as: pressure, temperature (absorption of gas) and a decrease when the liquid phase is supersaturated (desorption or gas leak).

Source
As a result, an increase in the concentration of a gas in the liquid medium or in the gaseous medium is generated.
Applications for aeration.
This process is extremely important in the treatment of wastewater, to separate undesired dissolved gases or to eliminate dissolved inorganic substances, by oxygenation, such as iron or manganese.
In smaller lakes and ponds, an imbalance of biological factors can easily occur, causing a drop in oxygen levels and making mechanical aeration necessary.
Insufficient water aeration, together with low levels of oxygen, can trigger fish mortality, unpleasant odors, and muddy waters. If a reservoir does not have sufficient oxygen circulation it is stratified with hot water on the surface and cold water with little oxygen in the bottom. The lack of oxygen increases the anaerobic decomposition of the sludge in the bottom. This increases the nutrients in the pond, which in turn causes the growth of algae. A pond or lake without enough oxygen circulation or aeration develops thermocline.
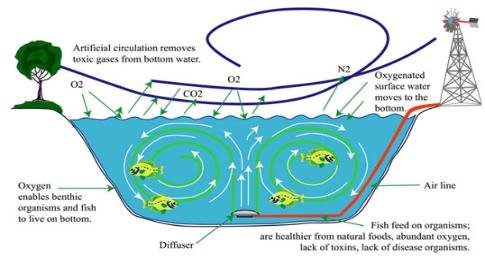
Source
Raw wastewater is aerated for a short space of time before treatment, in order to increase efficiency in subsequent operations

Source
In biological treatment processes air is used for two reasons: the first is the use of metabolic oxygen for the treatment of organisms and the second to ensure adequate mixing inside the chamber.
Flotation with air is useful to eliminate fats, solids and concentrate sludge.
Chlorine in gaseous form is used to achieve the objectives in the disinfection process. It is also common to add oxygen to the treated effluent after chlorination.
Importance.
In summary, aeration facilitates biological cycles in a pond and ensures the efficacy of bacterial nutrient-reducing and water-rinse products.
The amount of aeration needed for a pond or lake depends on its surface, depth, quality, and whether it accumulates runoff from fertilized areas. One (1) aeration horsepower per acre (4,048 m2) is the right amount for a common pond. If the pond is shallow or has an irregular shape, multiple small units are more effective than a large one. In general, the smaller the pond or lake, the greater the aeration needed per acre.
Influence of gas concentration.
If the water is exposed to a gas or a mixture of gases, there is a continuous exchange of gas molecules from the liquid phase in the gas phase and vice versa. As soon as the concentration of solubility in the liquid phase is reached, both gas currents will be of an equal magnitude such that changes in gas concentrations will not occur in both phases.
This dynamic equilibrium is generally referred to as the solubility or concentration of gas saturation in the liquid. The higher the gas concentration in the gas phase, the higher the saturation concentration in the liquid phase.
Influence of temperature.
When the gases are dissolved in the water, this process is usually accompanied by the release of heat. According to Le Chatelier's principle, increases in temperature result in reduced solubility.
Influence of impurities in water.
The distribution coefficient is valid only for pure water. Because water contains other constituents, these influence the solubility of gases and can be expressed by the activity coefficient (γ).
For pure water γ = 1, when γ increases the concentrations of dissolved substances in water are high which decreases the solubility, this influence of concentrations of impurities on the activity coefficient is represented by empirical formulas.
References.
- https://www.suezwatertechnologies.com/handbook/ext_treatment/ch_4_aeration.jsp
- http://info.oxymem.com/blog/why-is-aeration-important-for-wastewater-treatment
- http://www.purewaterproducts.com/articles/aeration-facts
- https://www.slideshare.net/preetibirwal007/gas-transfer

I learned a lot about aeration. Thanks a lot.
Great that's the goal, help other to learn new things
news that is very useful to read and provide knowledge, vote back and follow me @armanbpulo
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josalarcon2 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
thanks