Image a method that allows you to label a specific protein, one that is unique to a virus, bacterium or a disease like cancer or Alzheimer´s for destruction. Such a method has recently been developed and tremendous research efforts are being undertaken to bring this into maturity. This method utilized molecules that are called Proteolysis targeting chimeric molecules (PROTACs). PROTACs label a protein for destruction, using the body´s own destruction apparatus. Thus, PROTACs may have the potential to provide very specific treatment, with minimal risk of side-effects. They may even be a cure to cancer.
What Is A Protein
A protein can be almost anything. Proteins can have the form of a fiber or a ball. They can be used by your body to make energy, recognize malfunctions, control metabolic pathways or just help to turn sugar into energy. Proteins are vital to our body and make up a great portion of your body mass. We can eat proteins, but our body makes them itself – and all the time without a break.
Proteins are produced by transcribing a piece of DNA in the cell´s nucleus. Such pieces that are described as a unit are called a gene. However, DNA cannot be read by the cell organelle that produces proteins and thus needs to be transcribed. This is done by proteins (which assume the role of an enzyme) and results in a single-stranded piece of genetic material called RNA. In your body, the RNA is modified, so it can leave the nucleus and travel to the site of protein production without being degraded or altered (_ modification includes capping and formation of a tail, as well as cutting the RNA into units, in some cases_). The organelle that produces proteins is called the ribosome and can be found very close to the nucleus bound to a system of membranes called the endoplasmatic reticulum, or freely swimming around the liquid that fills the cell. The ribosome binds to the RNA and adds together amino acids, according to the recipe given in the RNA. This picture may clear up this process a little bit.
This results in a long chain of amino acids, the so called primary structure. Some amino acids can form a sort of bond with another amino acid that is based on attraction of a hydrogen atom to an area that is abundant with electrons (this is called a hydrogen bond, which is not really a bond). This results in a helix or sheeth-like structure, the secondary structure. Due to other effects like the hydrogen bond, as well as repulsion or attraction of certain amino acids to water (which is abundant in the cell liquid), the secondary structure folds, bends and assumes a 3D shape, the tertiary structure. Sometimes, multiple tertiary structures come together and form a quaternary structure. The function of a protein is dependent on its structure, thus the above process is vital. Since it is so important, protein production is closely observed by organelles in the cell. If a protein is found to be damaged or defect, it gets tagged for recycling.
How Does The Body Get Rid Of Unwanted Or Defect Proteins?
The discovery of the body´s regulation of proteins in the cell was rewarded with the 2004 Nobel Prize in Chemistry (1), which demonstrates the significance of this discovery. Our bodies are very efficient systems that try to recycle whatever possible. The same applies to proteins. Assume that a protein is faulty, due to a mistake in either transcription, translation or just in the formation of the tertiary or quaternary structure.
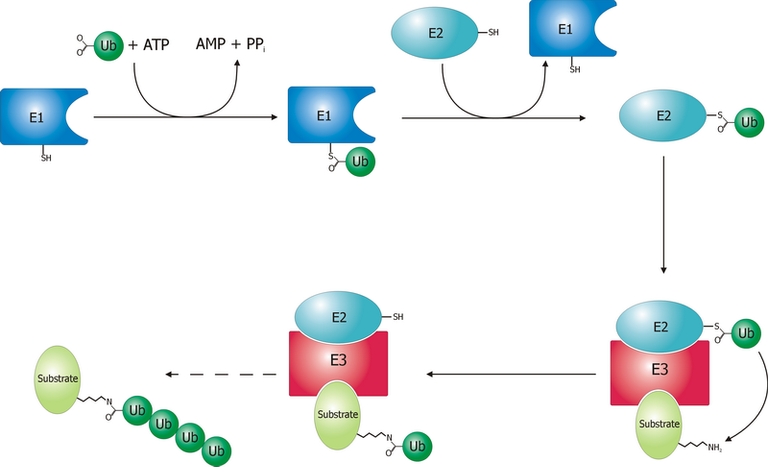
How do these proteins get tagged for recycling? There are three enzyme that are involved in the process of labelling. The first step, is the ATP-driven activation of a protein called ubiquitin by an enzyme called E1 (2). This leads to an activated ubiquitin molecule which can be used in the tagging process due to increased reactivity. The activated ubiquitin is then linked onto E2, another enzyme (2). E2 is important in the specificity of this system as certain E2 can function in conjugation with certain E3, which is the enzyme that makes this process most specific (2). E3 takes the ubiquitin that was bound to E2 and transfers that onto a protein (2).
There are many different types of E3, which shows how specific this machinery is (2). After these enzymes have added a chain of ubiquitins to a defect protein, it can get recognized by the protein-recycling center of the cell, the 26S proteasome. Here the protein gets unfolded, and then chopped into individual amino acids, which then can be used to make new proteins. (2), (3). In short, this is how the body breaks potentially harmful, defect proteins into useful, recycles amino acids with help of the proteasome.
How Do PROTACs Fit In This?
PROTACs are small molecules, with a simple structure. They consist of a part that can bind specifically to a protein, a linker, and a part that is specific for binding to an E3 (4). So a PROTAC can bind specifically to a protein, and then bind to an E3, which then ubiquinates the protein, tagging it for degradation in the proteasome. Personally, I think this is a very elegant approach.
Multiple PROTACs have been developed already. Some target cancers, for which we only have very limited therapeutic approaches such as prostate cancer (5). Other cancers can be targeted by PROTACs as well, by targeting specific receptors, that regulate transcription (6). We have a wide range of molecules that can specifically bind to proteins. Current research tries to identify more E3 specific molecules.
My Opinion
I wanted to write about this for a long time. The first time I heard about PROTACs was during my advanced biochemistry course, in which I had to read and present an article about PROTACs (_ we were randomly assigned an article from a list our professor made_). At that time I wanted to go into medical chemistry and I was very much intrigued by this technology.
While my paradigm has shifted a little bit and my interest for instrumentation has grown since I interned at a local biotech startup, I still believe that PROTACs offer promise to treat diseases that we were not able to. If we find a way to target several disease-specific proteins, we may be able to cure a lot of diseases that today can be a death sentence.
This company is working on the development of therapeutic PROTACs. They also offer informational materials if you are interested. I am also leaving you with a link to a google scholar search for PROTACs.
Shameless Self-Advertisement
Thank you very much for reading my post about PROTACs. If you have any questions please leave them in the comments. I also appreciate your opinions. Do you think this sounds like a promising new approach to curing diseases?
If you liked this post, leave an upvote. Also you may want to check out my series on instrumentation, my series Genius and Madness or my Lab Chronicles, in which I write about my research to develop filters that filter hormones out of water.
As always,
Cheers @lesshorrible!
.jpg)
Frankly speaking, Many of the terms went above my head. But, overall it's a detailed informative post. Great writing on this topic. I thank you for sharing such topics which are less shared in this platform, I believe.
I am sorry that I was unable to write it understandable enough. I will try to improve that in the future. Thank you very much for the encouragement @knowkrish. Cheers!
Dont be sorry. You have done quite well in writing such a lengthy and detailed post. I understand it took quality time for crafting out such a post. It's not your fault that I don't understand most of the terminology here.
Ok! But if you are curious what the terminology means, you can ask me or just check it out on the net. Thanks again! Cheers!
As artificial Intelligence comes of age we are gonna see Biotech actually be capable of understanding protein structure enough to understand the folding structures and compliment the disease's protein and make a complimentary match to target them. The next 10-20 years could be fairly huge for the war against diseases of all sorts.
Yeah, there is still a need to understand how the proteins assume their final configuration. The folding often requires molecular chaperones and other enzymes and immitating that system is very hard to do. But computer aided studies will help. Cheers!
That's pretty awesome, you could target the proteins in the plaques that form in the brains of Alzheimer's patients.
Yes @funbobby51, that is one of the hopes for the future. It is going to be interesting to see how this works out. Cheers!
Thank you for sharing this nice health talk.
You are welcome @adigunabiola! Cheers!
its really a very good information........for all of us,i wish it can be a big solution.....for cancer disease patient ..........@ i hope your research will be success...........
Thank you @abontikazaman! Sadly this is not my own research, it was started by researchers at Yale University. Cheers!
wow.. You really took lots of effot for this.
Thank you for the effort. Followed.
Hey @kinleytenzin! Thank you for acknowledging that! I am trying to write good content, and this was just something that I wanted to write about for a while. Cheers!
Nice post never heard about protacs until today...really wish that it really works so that cancer will be a thing of the past.
Watched a video talking about deficiency of vitamin B17 being one the major cause of cancer. How true is it? If you know about it, will love you to please throw more light
Hey @cyprianj, I really hope so too. Someone in my family died of cancer too early. Vitmanin B17 is apparently not even a vitamin. I heard that there are therapies where people take b17 supplements and eat a special diet, however, according to this website, it does not have scientific evidence suggesting it to be effective. Cheers!
Alright..thanks
Another great post! Protean tagging is a very promising area of research. I was curious if you had read anything concerning Superoxide dismutase concerning cancer therapy? I wrote my senior paper in college on the subject and i find it interesting the link between cancer and the rise of oxygen radicles on the body. superoxide dismutase has been linked to radiation therapy. If you get a chance look up deinococcus radiodurans and its ability to withstand massive doses of radiation due to magnanese superoxide dismutase .
Hey @csusbgeochem1. I have not read about this yet. However, funny enough, I worked with this enzyme. We meausred its concentration in a sample using inductively coupled plasma atomic emission spectroscopy. I just thought this was funny.
I will look that up, thank you for the suggestions. Cheers!
Hello Steemer, I feel honored to announce that you you have been awarded with a High Quality Content(7) “Badge Of Honor”. It is one of the most prestigious award a Steemer can get for writing good quality content on Steemit.
As a complimentary reward, SnapShare has upvoted and resteemed your post. Show your love for other steemers by upvoting this badge.
Know more about SnapShare at https://steemit.com/introduceyourself/@snapshare/what-is-snapshare-get-your-content-branded-with-snapshare.
Check out the meaning of various badges at: https://steemit.com/steemit/@snapshare/snapshare-badge-of-honor-which-one-did-you-get
If you are a reader viewing this badge, be assured that you’re reading a good post.
Congratulations!
Keep Steeming! Keep SnapSharing!
Cheers!
Hi there, this is an interesting post. Can you elaborate a little bit on how you think these factors could be used to fight cancer ?
While some cancer cells produce unique proteins (neoantigens) or normal proteins in abnormal quantities, how do you think degrading these proteins could kill the cancer cell ? (if we consider that most of proteins mutated in cancer are not essential for the survival of the cell because of the selection pressure)
This could also be very useful to fight prion diseases by promoting the degradation of abnormally folded proteins.
Hey @carlgbush! Thank you for your very thoughtful post and great questions. The current approach is to target receptors that are expressed in cancer cells. Here is a quote from an article:
I will also leave the link to this article in this post. http://www.pnas.org/content/113/26/7124.short
So it seems like you attack receptors that control transcription and thus, prevent the tumor from growing. Again, this research is still in its early stages. I tried to highlight its potential. I hope this answers your questions, otherwise let me know and I will go back and look for more studies. Cheers!
Thank your for the explanation and the link, that seems indeed promising for this type of cancer.
Cheers!
Thanks a lot for your research and the this really well explained and structured article!
I would love to read more about promising new medical/chemistry methods from you :)
Thank you @co-co! I will try to find some more then haha! Cheers!