When you are asked to write a "technical summary" and to use three sources "preferrably" scientific and you are still in grant proposal mode..... Glamorously overkilled this assigment.
Synthetic Biology - Using Engineering Approaches In Biology To Tackle Parkinson's Disease
Biotechnology has gained a lot of attention by the scientific community in the last
decades, as it has the potential to impact many areas from industry, to conservation, to
mining. (1-4) Advances in genetics and a better understanding of ecology and biochemistry
has enabled us to use plants and microbial life to develop new materials, (5) catalysts, (6-7) as
well as new methods to discover and produce pharmaceuticals such as penicillin,
teixobactin and insulin. (8-10) New developments in various technologies led to the emergence of a new category of biotechnology, which is called synthetic biology. Synthetic biology can be divided into two broader subgroups: creating artificial life and modifying existing life. (11) One aim of synthetic biology is to rewire and reprogram
organisms to tackle many of the arising challenges. (12) Synthetic biology, in more general
terms, is an engineering approach to biology. (13) This mini review will take a look at one
very recent article that deal with advances in synthetic biology. This article will be
summarized and analyzed and a conclusion about challenges and promises will be
drawn. A potential use of synthetic biology is in the engineering of biological carriers for
transport of therapeutics. Kojima et al. reported such a system using exosomes from
engineered mammalian cells. (14) Exosomes are believed to have potential as drug
delivery agents, due to their biocompability, bioavailability and ability to cross the blood-
brain barrier. (15-17) Kojima et al. anticipated to use designer exosomes as cell-based
theranostic agents. In short this means implanting cells into a patient that produce a
therapeutic agent, which is then transported to a specific site in the body. However,
there are certain challenges, such as poor efficiency of exosomal message and
transfer. (14) This paper aims to develop a novel design process, which may help to
overcome these challenges and to unleash the potential of designer exosomes in
treatment of Parkinson’s disease (PD) by transporting therapeutic mRNA into the brain.
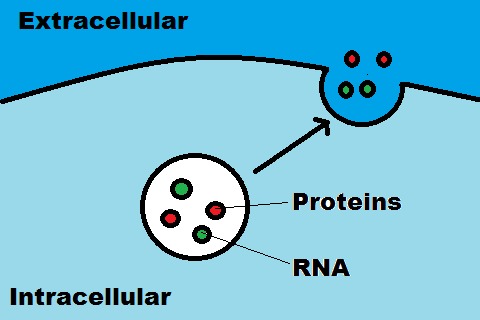
The first step in this project was to identify potential genes that would boost exosome
production. To achieve this, Kojito et al. fused a bioluminescence reporter (nanoluc (18) ) to
the C-terminus of a common exosome marker. (19,14) This reporter gene was fused into a
plasmid together with candidates for exosome production enhancement. Three genes
were identified that, when transferred to cells in tandem at a fixed ratio, enhanced the
luminescence measured in the supernatant by 15-fold to 40-fold. (14) In order to remove
masking effects, cell-culture supernatant was removed by stepwise centrifugation. (20) The enhancement of exosome production was also checked by direct quantification of exosomal proteins CD9 and TSG101, as well as nanoparticle-tracking analysis. This system was transferable to different cell lines, including human mesenchymal stem cells. The next step in this project was to design a practical application using this exosome production enhancement procedure. To develop a RNA packaging device, this team fused an archeal ribosomal protein (L7Ae) with the exosomal marker CD63 and added a C/D box into the 3’-untranslated region of the nluc gene. To help the exosomes to enter cells, they incorporated a gap junction protein, connexin 43, which is reported to enhance information transfer from exosomes to target cells via formation of a hexameric channel. (21) In order to target the exosomes to brain cells, they cotransfected a target molecule, RVG-Lamp2b, which binds to the nicotinic acetylcholine receptor. (22, 23) Without further exosome concentration, the supernatant was applied to HEK-293T cells which expressed the nicotinic acetylcholine receptor. The researcher found that strong luminescence in target cells was observed when all components were present. This
group then loaded their designed exosomes with therapeutic mRNA encoding catalase,
which decreases the cytotoxicity of reactive oxygen species (ROS). (24)
They found that their designer exosomes produced by engineered cells and loaded with mRNA for catalase did decrease the cytotoxicity of 6-hydroxydopamine (6-HD), which is used to trigger artificial PD. (25, 26) The next step then involved subcutaneous implantation of the engineered cell and verification that designer exosomes were present in the animal’s blood. Once this has been verified, the researcher investigated whether this implantation procedure would lead to delivery of designer exosomes into the brain of treated animals. This was also verified by improved activity in brain cells of animals that were treated with the engineered cells. Next, the scientists investigated whether their developed system could successfully attenuate neuroinflammation in mouse brains triggered by injection of 6-HD. To test this, mice were injected with the developed cells. In order to verify that possible attenuation was solely due to the designer exosomes carrying catalase mRNA, cells which lacked either the engineered transport system or catalase mRNA were also implanted to different animals. Attenuation of neuroinflammation was only observed in mice with transplanted cells that contained the engineered delivery system and catalase mRNA. This group has shown that they were able to optimize exosome production in engineered cells. Furthermore, they successfully developed a targeting-delivery system that transported therapeutic mRNA to the brains of mice. This system may find application in human patients with PD. However, there are still a few issues that need to be overcome. While the implantation of engineered cells led to attenuation of neuroinflammation due to 6-HD, the designer exosomes did also accumulate in the liver (27) and spleen, which are the major sites for clearing nanoparticles. (28) This may lead to toxicity or unwanted side-effects, which will potentially be a big problem in the development of a therapeutic application. Another issue that was not covered by this paper is the immune response of the treated organism to the implanted cells and the exosomes. While these issues need to be addressed in order to develop a therapeutic application of their designer exosome delivery method, this is a very promising step towards the application of synthetic biology in medicine. Additionally, this approach may be generalized and applied to other diseases. For example, these exosomes may be equipped with targeting agents for viruses or bacteria and thus create a new way of immunization for rare diseases. Furthermore, exosomes could be equipped with multiple therapeutic agents and become more effective by treating several causes of disease simultaneously. A great advantage of this approach, in comparison to other nanoparticle-based drug-delivery systems, is that it is entirely biology based. Many potential therapeutic nanoparticles are based on metals. However, metals may be harmful to the body and they are more expensive and less permanent than the theranostic approached outlined in this paper. I have not read about similar approaches before and it was very interesting to read about applications of microbiology that go beyond the production of enzymes or applications in food industry.
Sources
(1) Krauss, U.; Jaeger, V.D.; Diener, M.; Pohl, M.; Jaeger, K.E. “Catalytically-active
inclusion bodies – Carrier-free protein immobilizates for application in biotechnology and
biomedicine” J. Biotechnol. 2017, 258, 136-147 DOI: 10.1016/j.jbiotc.2017.04.033
(2) Thompson, C.C.; Kruger, R.H.; Thompson, F.L. “Unlocking Marine
Biotechnology in the Developing World” Trends Biotecchnol. 2017, 35, 1119-1121 DOI:
10.1016/j.tibtech.2017.08.005
(3) Corlett, R.T. “A Bigger Toolbox: Biotechnology in Biodiversity Conservation”
Trends Biotechnol. 2017, 35, 55-65 DOI: 10.1016/j.tibtech.2016.06.009
(4) Dunbar, W.S. “Biotechnology and the Mine of Tomorrow” Trends Biotechnol.
2017, 35, 79-89 DOI: 10.1016/j.tibtech.2016.07.004
(5) Chen, L.; Zou, M.; Hong, F.F. “Evaluation of Fungal Laccase Immobilized on
Natural Nanostructured Bacterial Cellulose” Front. Microbiol. 2015, 6
DOI: 10.3389/fmicb.2015.01245
(6) Suzuki, K.; Hirai, H.; Murata, H.; Nishida, T. “Removal of estrogenic activities of
17β-estradiol and ethinylestradiol by ligninolytic enzymes from white rot fungi” Water
Res. 2003, 37, 1972-1975 DOI: 10.1016/S0043-1354(02)00533- X
(7) Beck, S.; Berry, E.; Duke, S.; Milliken, A.; Patterson, H.; Prewett, D.L.; Rae,
T.C.; Sridhar, V.; Wendland, N.; Gregory, B.W.; Johnson, C.M. “Characterization of
Trametes versicolor laccase-catalyzed degradation of estrogenic pollutants: Substrate
limitation and product identification” Int. Biodeterior. Biodegrad. 2018, 127, 146-159
DOI: 10.1016/j.ibiod.2017.11.020
(8) Chain, E. “The early years of the penicillin discovery” Trends Pharmacol. Sci.
1979, 1, 6-11 DOI: 10.1016/0165-6147(79)90004- X
(9) Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.;
Mueller, A.; Schaeberle, T.F.; Hughes, D.E.; Epstein, S.; Jones, M.; Lazarides, L.;
Steadman, V.A.; Cohen, D.R.; Felix, C.R.; Fetterman, K.A..; Millett, W.P.; Nitti, A.G.;
Zullo, A.M.; Chen, C.; Lewis, K. “A new antibiotic kills pathogens without detectable
resistance” Nature, 2015, 517, 455-459 DOI: 10.1038/nature14098
(10) Johnson, I.S. “Human Insulin from Recombinant DNA Technology”Science,
1983, 219, 632-637 DOI: 10.1126/science.6337396
(11) Benner, S.A.; Sismour, A.M. “Synthetic Biology” Nature Reviews Genetics,
2005, 6, 533-543 DOI: 10.1038/nrg1637
(12) Khalil, A.S.; Collins, J.J. “Synthetic biology: applications come of age” Nature
Reviews Genetics, 2010, 11, 367-379 DOI: 10.1038/nrg2775
(13) Serrano, L. “Synthetic biology: promises and challenges” Mol. Syst. Biol. 2007, 3,
DOI: 10.1038/msb4100202
(14) Kojima, R.; Bojar, D.; Rizzi, G.; Charpin-El Hamri, G.; Daoud El-Baba, M.;
Saxena, P.; Auslaender, S.; Tan, K.R.; Fussenegger, M. “Designer exosomes produced
by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease
treatment” Nature Communications, 2018, 9 DOI: 10.1038/s41467-018- 03733-8
(15) Tan, A.; Rajada, J.; Seifalian, A.M. “Exosomes as nano-theranostic delivery
platforms for gene therapy” Adv. Drug Deliv. Rev. 2013, 65, 357-367
(16) Van der Meel, R. et al. “Extracellular vesicles as drug delivery systems:
lessons from the liposome field” J. Control. Release, 2014, 195, 72-85
(17) Ha, D.; Yang, N.N.; Nadithe, V. “Exosomes as therapeutic drug carriers and
delivery vehicles across biological membranes: current perspectives and future
challenges” Acta Pharm. Sin. B, 2016, 6, 287-296
(18) Hall, M.P. et al. “Engineered luciferase reporter from a deep sea shrimp
utilizing a novel imidazopyrazinone substrate” ACS Chem. Biol. 2012, 7, 1848-1857
(19) Suetsugu, A. et al. “Imaging exosome transfer from breast cancer cells to
stroma at metastatic sites in orthotopic nude-mouse models” Adv. Drug Deliv. Rev.
2013, 65, 383-390
(20) Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. “ Isolation and
characterization of exosomes from cell culture supernatants and biological fluids” Curr.
Protoc. Cell Biol. 2006, 30 DOI: 10.1002/0471143030.cb0322s30
(21) Soares, A.R. et al. “Gap junction protein Cx43 is involved in the
communication between extracellular vesicles and mammalian cells” Sci. Rep. 2015, 5
(22) Alvarez-Erviti, L. et al. “Delivery of siRNA to the mouse brain by systemic
injection of targeted exosomes” Nat. Biotechnol. 2011, 29, 341-345
(23) Hung, M.E.; Leonard, J.N. “Stabilization of exosome-targeting peptides via
engineered glycosylation” J. Biol. Chem. 2015, 290, 8166-8172
(24) Haney, M.J. et al. “Exosomes as drug delivery vehicles for Parkinson’s
disease therapy” J. Control. Release, 2015, 207, 18-30
(25) Bernstein, A.I.; Garrison, S.P.; Zambetti, G.P.; O’Malley, K.L. “6-OHDA
generated ROS induces DNA damage and p53- and PUMA-dependent cell death” Mol.
Neurodegerner. 2011, 6
(26) Saito, Y. et al. “Molecular mechanisms of 6-hydroxydopamine- induced
cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -
independent action” Free Radic. Biol. Med. 2007, 42
(27) Wiklander, O.P.B. et al. “Extracellular vesicle in vivo biodistribution is
determined by cell source, route of administration and targeting” J. Extracell. Vesicles,
2015, 4
(28) Barile, L. Vassalli, G. “Exosomes: therapy delivery tools and biomarkers of
diseases” Pharmacol. Ther. 2017, 174

This was a "little" Microbiology assigment I had to write over the weekend. Obviously, I completely overdid it and at the end I felt I had written something that is good enough to share. Not to mention that I am bored of this class because it is relatively easy. After all, I am just a dickhead trying to impress the biology majors.
As always,
Cheers @lesshorrible!

Great piece @lesshorrible. Biotechnology is really fascinating. It is an aspect of science that i really appreciate too. It would soon become a very common practice to use engineered cells and even the designer exosomes highlighted here, to treat various forms of degenerative diseases. Thanks for sharing. And I'm quite sure you'd ace the assignment.
This was the first time I read about designer exosomes so I was actually really excited about this - nerd... I also figured out that Nature has free publications, which was the bigger find. Thank you for your comment. Cheers!
Congratulations @lesshorrible! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
To support your work, I also upvoted your post!
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last announcement from @steemitboard!
Congratulations @lesshorrible! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPHey bro quick tip!
If your going to introduce something like a cure to diseases you should show via text and or pic the symptoms first so people will get alarmed by it!
Hey thanks for the tip. I am assuming that most people are aware of PD and it is difficult to show PD symptoms since most of them are motion associated (tremor, robot-like movements, stiffness, etc.) Thank you for your comment! Cheers!
Yuppe!
Parkinson's Disease is well known, easy to Google, and @lesshorrible is a more experienced Steemian than you are, so give him a break. He knows what he is doing.
Leading by fear is the wrong approach for a scientific paper, where the goal is objectivity, and accuracy. Fearmongering in this context is entirely inappropriate.