Many inventors are trying to find that one reaction, that one process that will make them rich and famous. There are a few "jackpots" out there you can hit. However, as often is the case in science, it is very difficult to overcome the problems that are present, and the challenge is to use established knowledge in new ways. I want to introduce to you a long standing problem, that has an industrial significance. I am speaking of nitrogen fixation.
Why Do We Care About Nitrogen Fixation
Nitrogen is very important for industry, agriculture, and nature. Plants need nitrogen to grow (nitrogen is a significant part of fertilizers), and industry has various applications for it (for example in production of ammunition). Our air is composed of roughly 80% nitrogen. So it is very abundant and readily available? No!
The problem with atmospheric nitrogen is, that it is present in its standard state. Nitrogen forms homoatomic molecules that consist of two atoms of nitrogen. These two atoms form a very strong chemical bond, a tripple bond, making atmospheric nitrogen very lazy when it comes to chemical reactions.
Fritz Haber - Source
It was not until Fritz Haber invented the Haber-Bosch cycle that we were able to forge atmospheric nitrogen into the more useful form ammonia (one nitrogen atom bound to three hydrogen atoms). The invention of the Haber-Bosch cycle had global implications.
Indeed, nearly half of the existing human population could not exist without application of the Haber − Bosch process for production of nitrogen fertilizers
Source
In the Haber-Bosch cycle, atmospheric nitrogen, together with hydrogen molecules, gets introduced into a high pressure, high temperature system. With the help of catalysts (materials that essentially enable reactions to occur under more favorable conditions) this process is able to bind nitrogen into ammonia.
This is a very energy intensive process and great effort is dedicated to find procedures that occur under more "ambient" conditions (1). The energy need of the Haber-Bosch cycle is on a global scale.
Ammonia produced via the Haber-Bosch process; at a rate of 275 billion lb per year; and its derivative products ammonium nitrate and urea are among the highest volume chemicals currently made. This one process consumes up to 5% of the world's annual natural gas production to make hydrogen and generate heat to run the reaction, and it consumes about 2% of the world's annual energy production.
Source
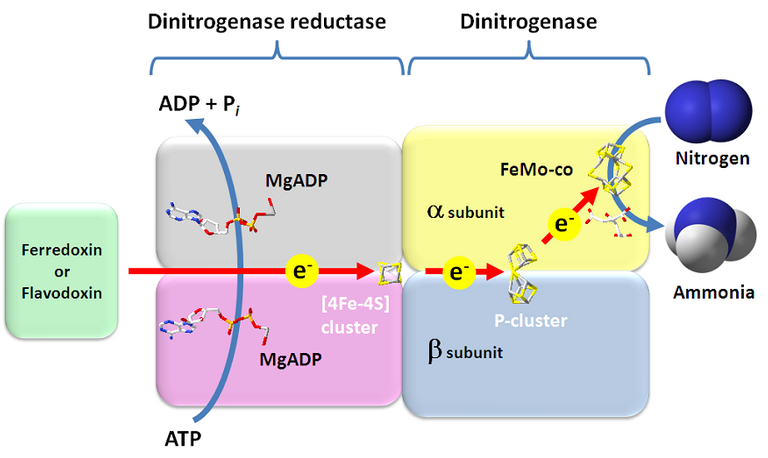
This is a major burden, especially when we consider that the natural resources we have a shrinking and energy consumption is increasing with increasing population. It is obvious that there is a need for more energy-efficient processes to fix nitrogen. Here nature serves as our inspiration. Many organisms are able to fix nitrogen under ambient conditions with an enzyme called nitrogenase.

Peanut, a carbon-fixing plant - Source
How May We Solve The Problems Of The Haber-Bosch Cycle Using Biotechnology?
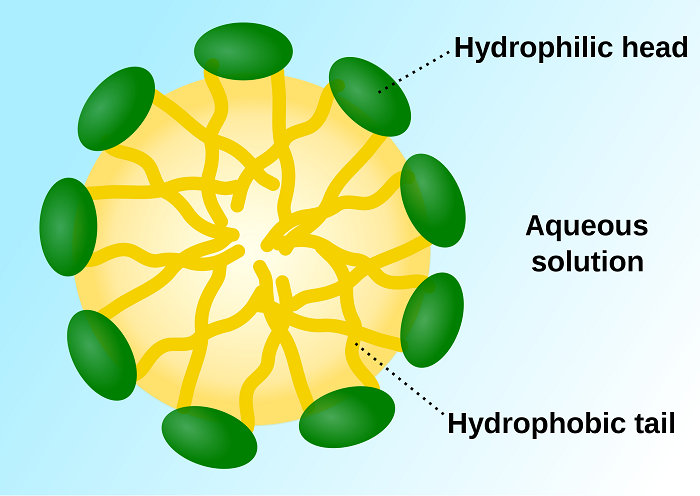
As I am an over-ambitious undergraduate student, I wanted to single-handedly solve this problem. I thought that I may be able to find a way to produce fixed nitrogen as I have a fresh look on things. My goal was to create artificial micelles (micelles are little spheres that form when dropping a liquid into another liquid and they are not miscible) and fill them with nitrogenase.
The micelles were supposed to localize the nitrogenase enzymes and to prevent them from being exposed to oxygen, which can cause irreversible deactivation (basically destruction) of the enzyme (2). Sadly even the concentration of oxygen dissolved in water is too high for nitrogenases to work. Needless to say that this demonstrated to me beyond any doubt that I am nowhere near the scientific elite. So I aborted the mission.
Luckily, there are smarter people out there who are trying to find a way to yield the potential of nitrogenases. It seems possible to fix nitrogen into ammonia using nanosheets composed of Bismut, Oxygen and Bromine, using water and visible radiation (visible light)(1). The process that enables this to work goes beyond the scope of my post but I recommend checking it out if you have a background in inorganic and physical chemistry. In short, this technique is mimicing charge transfers and enzyme complexes that are present in nitrogenases.
Another approach is to alter nature. The introduction of nanocrystals comprised of cadmium sulfide into cells with nitrogenase seems to enable those cells to perform nitrogen fixation without the need of ATP hydrolysis (ATP is the "energy currency" in almost every living organisms, and the hydrolysis of ATP provides energy for other chemical reactions to occur
Another interesting idea is to design approaches that would decrease our need for nitrogen from fertilizers, thus allowing to downscale the Haber-Bosch cycle. This paper discusses several possible approaches to either design nitrogen-fixing bacteria that are able to form a symbiontic relationship with crop plants (wheat, rice, etc.). Another idea is to modify crop plants genetically to enable them to fix nitrogen themselves, thus basically making them independent from fertilizers. Sadly this is a very complicated process and not yet realistic, as stated in above paper.), using energy from light reaching 63% of the efficiency of the natural ATP-dependent process. [(3])(http://science.sciencemag.org/content/352/6284/448).
My Opinion
We still have ways to go if we want to be less dependent on the Haber-Bosch cycle and have more sustainable ways of producing fixed nitrogen. Personally, after my research into this topic, I believe that the solution may not be a single one, but a vast amount of alternative procedures.
There is a need to increase the production of fixed nitrogen, because we are probably not able to maintain the population of the planet without fertilizers. However, there is only so much energy we can produce and it is vital for the future to find alternative, sustainable ways to produce fixed nitrogen. However, as a chemist and future scientist, I believe that people smarter and more experienced than I am will come up with solutions.
With the rise of synthetic biology we may be able to design entirely new organisms that are able to fix nitrogen and efficiently produce ammonia. But that is just a vision and very far away from what is realistically doable right now.

Trichodesmium, a carbon fixing algae - Source
Shameless Self-Advertisement
Thank you very much for reading this lengthy post. I know there is probably a lot more to talk about (like electrochemical nitrogen fixation) but I am personally more passionate about biotechnological approaches to this issue because they offer a more renewable approach.
I will appreciate your comments and questions. If you have any, I will read them all and comment, unless it is a bogus "thank you for" comment. I have put in a lot of time and effort into writing this post and it would be great if you could at least respect the effort.
If you like my work, please do not hesitate to check my blog. Also, upvote and resteem if you liked this!
As always,
Cheers @lesshorrible!
I agree with you that fertilisers are a huge issue. The run off causes massive algea blooms that deplete the oxygen in the water killing marine life. The only issue is by designing plants on a large scale to pull nitrogen from the air then your are reducing the nitrogen ¨buffer¨ out atmosphere uses to keep us alive. We only require a small mix of oxygen and higher concentrations are deadly.
Yeah, there is definitely a need to investigate this. It seems like we are reaching a point where we need to make changes in the way we live and produce. I think Elon Musk and Stephen Hawking have a point, there may be a need for us to start migrating into space (or find another habitat). Cheers!