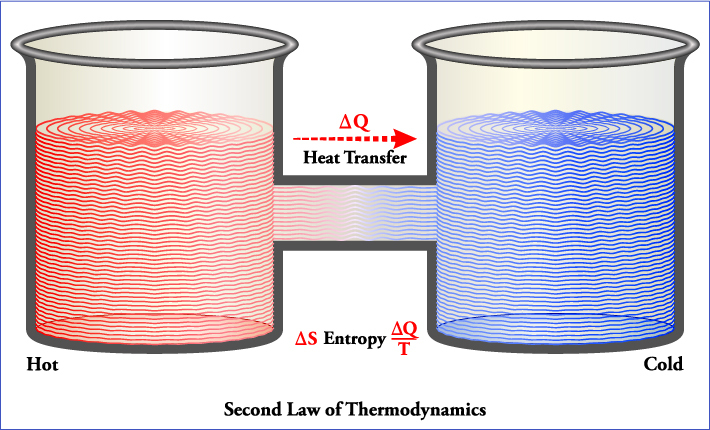
The first law of thermodynamics and the principle of conservation of mass describe the conservation of a particular property. The first law expresses the form in which the energy of the system is altered due to the transfer of energy by the system borders and mass transport inside and outside of said system. The First law is a rigorous accounting procedure that describes the changes of the energy of the system. The conservation of the mass quantifies the changes of the mass in the system. Both conservation statements directly relate the change of a property of the system with the transfer by a border. The second law of thermodynamics also relates a property of the system to the energy transfer across borders, but the relationship simply specifies the direction of the change.
The first law states that energy is conserved in any process and does not impose any restriction with respect to the direction in which it occurs. However, experience indicates the existence of this restriction, whose formulation completes the foundations of thermodynamics and whose concise expression constitutes the second law. The differences between the two forms of energy, heat and work, provide some light on the second law. In an energy balance, heat and work are included as simple additive terms, which implies that a unit of heat, that is, a joule, is equivalent to the same unit of work. Although the above is valid for the energy balance, experience shows that there are differences between heat and work in terms of quality. This experience is summarized in the following facts.
Work is quickly transformed into other forms of energy; for example, in potential energy when lifting a weight, in kinetic energy by accelerating a mass or in electrical energy by the operation of a generator. These processes can be performed with a conversion efficiency close to 100 percent when friction is eliminated, which is a dissipative process that transforms the work into heat. In fact, the work is completely transformed in heat, as Joule's experiments showed.
Enunciations of the second law
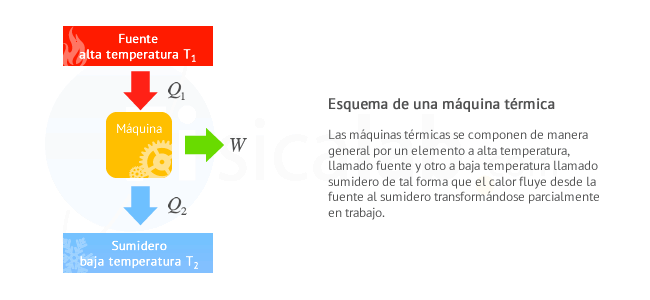
The observations described above are the result of the restriction imposed by the second law on the directions in which the real processes occur. It is possible to formulate many statements that describe this restriction and that, therefore, serve as enunciations of the second law. Two of the most common are the following:
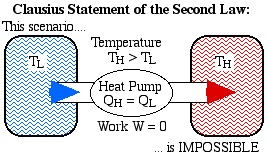
No equipment can operate in such a way that its only effect (in the system and its surroundings) is to completely convert all the heat absorbed by the system into work done by the system.
There is no process that consists exclusively in the transfer of heat from one temperature level to a higher one.
The first statement does not affirm that heat can not become work; The only thing that the process can not do is leave both the system and its surroundings without any change. Consider a system formed by an ideal gas contained in a piston cylinder that expands reversibly at a constant temperature.
Entropy
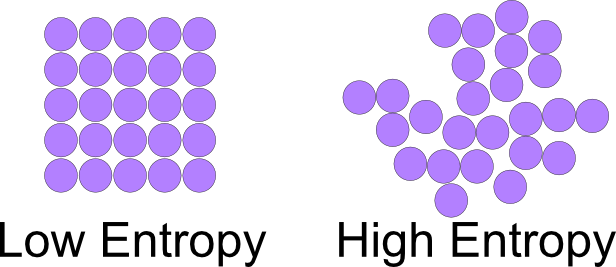
The second principle of thermodynamics is not limited exclusively to thermal machines but deals, in general, with all the natural processes that occur spontaneously. We can say that it deals with the natural evolution of thermodynamic systems, that is, the direction in which they advance. This direction is associated with the internal molecular distribution of the molecules. To study the spontaneity of processes, the Austrian Ludwig Edward Boltzmann introduced a new magnitude called entropy.
It is a physical quantity that for a thermodynamic system in equilibrium measures the number of microstates compatible with the equilibrium macrostate, it can also be said that it measures the degree of organization of the system, or that it is the reason for an increase between internal energy as opposed to an increase in system temperature.
CARNOT MACHINE.
The Carnot cycle (Sadi Carnot, French, 1796 - 1832), is of great importance from the practical point of view as theoretical. Carnot demonstrated that a thermal machine that operated in an ideal reversible cycle between two heat sources would be the most efficient machine possible. An ideal machine of this type, called the Carnot machine, sets an upper limit on the efficiency of all machines. This means that the net work done by a work substance carried through a Carnot cycle is the maximum possiblefor a given amount of heat supplied to the working substance. Carnot's theorem is stated as follows:
No real thermal machine operating between two heat sources, can be more efficient than a Carnot machine, operating between the same two sources.
For more information consult the bibliography or links that I leave here:
Thank you very much for your support, I will continue with my work
Congratulations @martinezkarla! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP