Energy is a term widely used even when it represents a very abstract term, for example when someone feels tired usually say that they have no energy, it is common to read about the need to find alternatives to non-renewable energy sources. Unlike matter, energy is known for its effects can not. In general, energy is usually defined as the ability to do a job. All forms of energies are capable of doing work, that is, exerting a force at a distance, but not all are important when talking about chemistry or physics, for example, chemists usually define work as the change of energy produced by a process. The kinetic energy, which is produced by an object in motion, is the one that is studied with greater emphasis by physicists.

All forms of energy can be converted into one another. When you are under the sun you feel heat because in the skin the radiant energy is converted into thermal energy. When you exercise, the chemical energy stored in the body is used to produce kinetic energy. When a ball starts rolling down a hill, its potential energy becomes kinetic energy. No doubt you can think of many examples. Scientists have come to the conclusion that, although energy can be in different ways than convertible energy, energy can not be created or destroyed. When a form of energy disappears appear another of the same magnitude and vice versa. This principle is summarized in the law of conservation of energy. The total energy of the universe remains constant.
Thermochemistry
Thermochemistry comes from the Greek thermos which means heat and chemistry consist of the study of the transformations that heat energy undergoes in chemical reactions, emerging as an application of thermodynamics to chemistry. Frequently we can consider that chemical reactions occur at constant pressure (open atmosphere, that is, P = 1 atm), or at constant volume (that of the receptacle where they are being made).
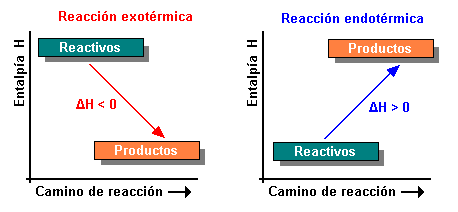
The reactions where the variation of enthalpy is positive (heat absorbed in the reaction) are called endothermic reactions, while those whose enthalpy variation is negative (heat is given by the system during the reaction) are called exothermic reactions. If the endothermic reaction takes place in a system with adiabatic walls, as a consequence of the reaction, a decrease in the temperature of the system occurs. If the reaction is exothermic and is carried out in a container with adiabatic walls, the final temperature of the system increases. If the walls of the system are diathermic, the System Temperature remains constant regardless of the energy transfer that occurs due to the change in composition.

This part of the Chemistry is the object of study of the Thermochemistry, to which we can define how the branch studies the changes of caloric energy that accompany the chemical reactions. When the heat of reaction is determined, the amount of heat released or absorbed in a reaction at the same temperature of the reactants is known. If the chemical reaction produces energy absorption it is called endothermic, and if there is, the opposite, energy release is called exothermic.
Status functions
They are state variables that have a unique value for each state of the system. Its variation only depends on the initial and final state and not on the developed path. They are functions of the state: Pressure, temperature, internal energy, enthalpy. They are NOT: heat, work.
Enthalpy
Enthalpy is the amount of heat energy of a substance. In a chemical reaction, if the enthalpy of the products is less than that of the reactants, heat is released and we say that it is an exothermic reaction. If the enthalpy of the products is greater than that of the reactants, heat is taken from the medium and we say that it is an endothermic reaction.

Types of reactions according to the Enthalpy:
Endothermic reactions
Are those reactions where heat is absorbed, this means that the energy of the molecules of the resulting substances or products (EP) is greater than the energy of the molecules of the reactants (RE). The medium where this type of reaction occurs cools.
Exothermic reactions
They are those reactions where heat is released, this means that the energy of the molecules of the resulting substances or products (EP) is less than the energy of the molecules of the reactants (RE). The medium where this type of reaction occurs is heated.
Entropy
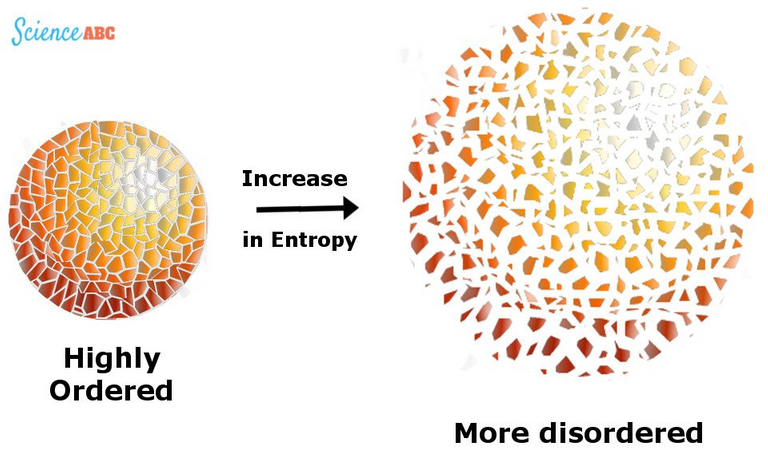
Entropy can be the thermodynamic physical quantity that allows us to measure the non-usable part of the energy contained in a system. This means that this part of the energy cannot be used to produce a job. Entropy is also understood to be the measure of the disorder of a system. In this sense, it is associated with a degree of homogeneity. The entropy of formation of a chemical compound is established by measuring the one that conforms to each of its constituent elements. The greater the entropy of formation, the more favorable its formation will be. In the theory of information, entropy is the measure of the uncertainty that exists before a set of messages (of which only one will be received). It is a measure of the information that is necessary to reduce or eliminate uncertainty.
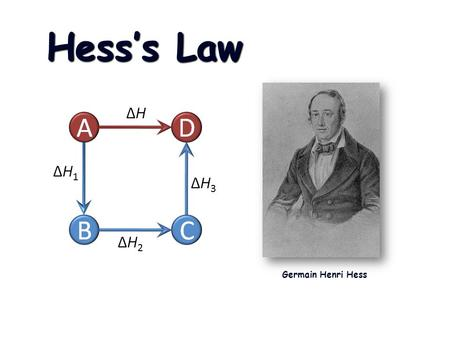
The law of Hess
The law of Hess in thermodynamics is used to indirectly check the heat of reaction, and according to the forerunner of this law the Swiss chemist Germain Henri Hess in 1840 institutes that, if a process of reactants react to give a process of products, the heat of The reaction released or absorbed is independent of whether the reaction is carried out in one or more periods. That is to say, that the heat of reaction only needs the reactants and the products, or also that the heat of reaction is a function of state. Hess dealt totally with chemistry and one of the best known works was the Law of Constant Sum of Heat, which was later named as Hess's Law in his honor; mainly explained that the enthalpy of a reaction could be achieved by algebraically adding the enthalpies of other reactions some linked with the one that matters. The Law of Hess is the use of chemical reactions becoming one of the first principles of thermodynamics. This principle is a closed adiabatic system, that is to say, that there is no exchange of heat with other systems or their environment as if it is isolated, which develops in an initial phase to another final phase.