Most portable electronics use some sort of battery for power storage. Things like phones and computers will use rechargeable batteries, almost all always lithium-based. Other items like TV remotes and flashlights use disposable batteries, namely AA's and AAA's in most cases.
Usually these single-use batteries are alkaline or carbon-zinc based, and typically they can't be recharged (although rechargeable AAA's obviously exist). But have you heard of the aluminum-air battery? This device is a very efficient, high energy storage battery that stores energy via a reaction between ordinary aluminum metal (found in foil, cans, and many structures) and ordinary O2 (Oxygen) gas found in the air all around the planet.
I've also whipped up a quick Al-Air battery as a demonstration for you to see. I'll go over that at the end once I've covered the background.
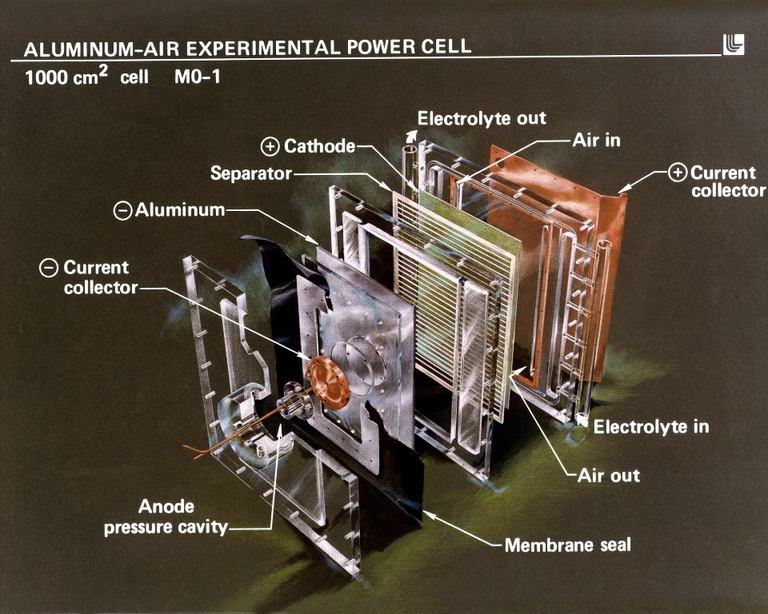
Example of an Al-Air battery design
Credit
Quick Review of Batteries
Let's first briefly discuss how batteries themselves work before going into the specific of the Al-Air battery. All batteries produce electricity via a chemical reaction between two or more compounds. In a way, single-use disposable batteries are electric generators, producing electrical energy from chemical potential energy stored in the respective compounds within the battery.
A common disposable battery type (that's starting to die out in favor of alkalines) we can use as an example are Zinc-Carbon batteries. In this cells, a reaction takes place between zinc metal and manganese oxide. This produces a voltage difference which you can use to drive current and power things. In this case, the "carbon" in the battery name refers to a carbon anode (+), used to extract charge from the reaction. Here's a Zinc Carbon battery:
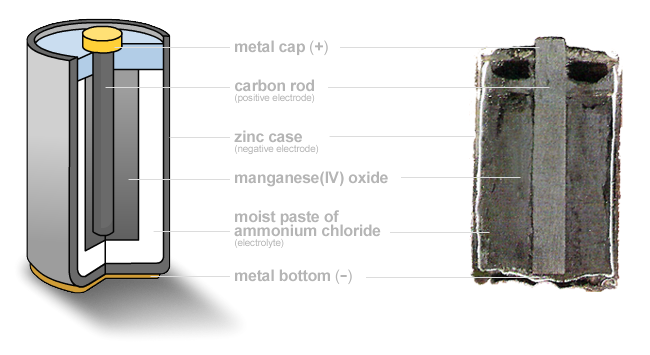
Zinc-Carbon battery. I only still see these at dollar stores, as most expensive batteries have switched to alkaline.
Credit
In general, batteries consist of an anode (the positive terminal) made of one material, a cathode (negative terminal) made of another material, and an electrolyte. The two materials react to produce the voltage difference. The electrolyte allows charge to flow between the two electrodes and facilitate the reaction, and typically takes the form of a liquid with dissolved ions in it - salt water works as an electrolyte, for example. The electrolyte doesn't usually directly participate in the chemical reaction - rather, it facilitates it, and allows current to flow across the battery when you hook up, say, a light to the cell.
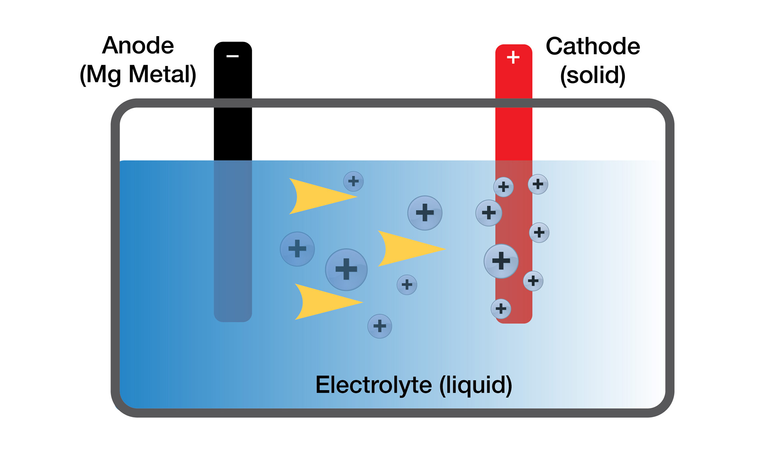
Example of a battery
Credit
Different battery materials/chemistry provide different results. Some, like the lead-acid batteries found in cars, are meant to withstand large temperatures ranges and produce a lot of current. Others, such as alkalines and the Al-Air batteries we are going to discuss next can store more energy in a smaller form factor.
The Aluminum-Air Battery
Now that I've covered batteries a bit, on to the topic of the post!
Aluminum-Air batteries use the two materials in their name to produce a flowing electrical current. In this case, Aluminum forms the anode (positive), and air forms the cathode (negative). By air here, we are really referring to oxygen gas - O2, that is. Several sub-reactions occur in these batteries, but the overall reaction has the form:
4 Al + 3 O2 + 6 H2O → 4 Al(OH)3
That is, aluminum, oxygen, and water combine to form aluminum hydroxide. The end result is a voltage is produced across the anode and cathode. In the case of Al-Air batteries, the best you can hope for is 1.2 Volts per cell. In practice, this voltage will depend on how you design the battery, as you'll see below. Practically speaking, you don't actually use the air as the cathode, you use something like carbon (just like in zinc carbon batteries) to collect the current.
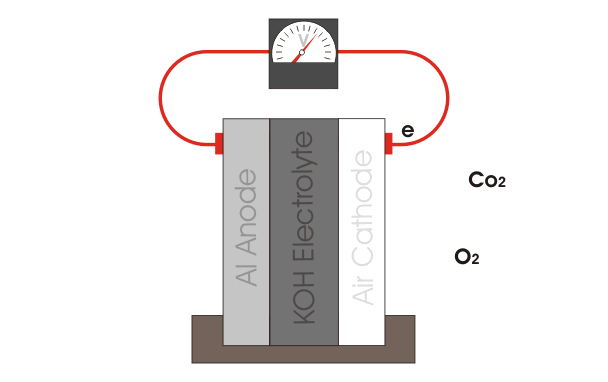
Al-Air Battery Illustration
Credit
Al-Air batteries are some of the best batteries available for energy density (the amount of electrical energy you can store in a given mass). This makes them useful when small batteries and high energy usage are needed - say, an electric car. The big downside is that they can't be recharged, although you can just swap out the aluminum anode to "recharge" the battery. This essentially lets you build a car powered by aluminum metal as fuel.
You know how in Back to the Future 2 they put the can into the car to make it run? Well, this isn't a fusion reactor, but the end result is the same, as you could literally power something using only aluminum cans as fuel.
In practice, Al-Air batteries can achieve energy density of around 1300 Whr per kilogram. That probably doesn't mean much to you, but this is huge: Car lead-acid batteries can only get around 40 Whr per kilogram. This means that an aluminum air battery of a given size can provide 32 times more power than a car battery of the same size. This means that a one kilogram aluminum air battery could power your phone for weeks on end, and could then recharged by swapping out the corroded aluminum chunk serving as the anode.

With Al-air batteries, you can use cans as fuel for electricity. I'm not sure how much energy each can could produce, but it's probably not a lot.
Credit
Certain engineering issues prevent Al-air batteries from taking over all of our disposable power needs. However, the potential is enormous. Imagine, instead of filling up your car at the gas station, you purchased a piece of aluminum metal. When you need to fill up (which would happen much less frequently than with gas-powered cars), you just pop the hood, pull out your old aluminum anode (now mostly aluminum hydroxide) and slot in the new piece of aluminum, and you're good to go another thousand kilometers. Now whether or not this would solve pollution issues or be inexpensive relies on how efficient it is to produce the aluminum anodes, but the fact that aluminum-powered cars are at least somewhat possible seems pretty cool to me. The huge power density of Al-air batteries is what makes this actually feasible compared to other battery types.
Practical Example: 30-second DIY Al-Air battery
Since I've done this before to experiment with homemade batteries, I decided to put together a quick aluminum air battery to show you before writing this post.
You can make a very awful, very crude aluminum air battery by taking a small piece of aluminum foil and placing a paper towel on top. Wet the paper towel, sprinkle some ordinary table salt on top (or baking soda), and there you have it: A simple Al-air battery.
If you take this soggy device and connect a multimeter across it (one lead on the aluminum, one lead on the paper towel) you will see a significant voltage, indicating that your battery is working and a reaction is taking place:

As you can see, we are producing around 350 millivolts (0.35 Volts) from our aluminum-air reaction. The quarters are only here to press down the paper towel into the foil and don't contribute to the reaction. You can just double this voltage by actually making an effort to press the paper towel completely into the foil and uniform ally soaking the paper towel in salt water. You can actually make a lot of batteries like this using coins and what not, but the aluminum air one is pretty cool because it uses ... air.
Don't even think about powering anything with this DIY device, though. Any attempt to do this would immediately drain the battery, since very little aluminum is available for reactions, not to mention salt is kind of a bad electrolyte for this and the overall battery is very small to begin with. However, with more aluminum (perhaps a can?), a better setup, and a carbon current collector, you could definitely build a nice DIY aluminum air battery that could power something using the awesome power of ... aluminum.

Here's a decent DIY aluminum air battery, that I didn't make, that can actually light up a bulb. This one uses a carbon current collector, and could actually be useful. However, it has the downside of not taking 30 seconds like mine does. Click the credit link below to see this device.
Credit
It'd be really cool to figure out how to make your own machine that would power phones and the like using old aluminum soda cans, made simple to the point where you can just drop the cans in. I don't know if the cans would provide enough aluminum to be useful, though. Maybe a project for another day.
And that's the end of this post on some of the best single-use batteries you can make. Aluminum-air batteries are honestly quite powerful considering they are made from an extremely common, cheap metal and the literal air around you. Personally, I'd really like an aluminum powered car, although I expect that electric rechargeable cars will become affordable long before aluminum-air cars are for sale, so I wouldn't get your hopes up.
I hope you were able to learn something new! Let me know if I said anything incorrect, or if you have questions/comments.
Thanks for reading!
Sources for additional reading
Could aluminum replace gasoline?
Aluminum-air battery Wikipedia Entry
Zinc-carbon battery Wikipedia Entry
An inexpensive paper-based battery
Electric test car with aluminum-air battery takes to the track
Images not credited are my own. You are welcome to use them with credit.
I concur an interesting engineering post. But I do see a few problems like
energy density(edit: I see you mentioned 1300 Whr/kg for aluminium compared to 160 Whr/kg for a Tesla Li Ion battery) and the fact that Aluminium takes huge amounts of electricity to produce. Having worked in an Aluminium smelter I can attest to that, the currents are so strong that the induced field near busbars exert noticeable force on steel cap boots as you walk!Of course with recycled Aluminium this is not an issue...
Aluminum is a readily available metal and this really proves that there is soon to be yet another breakthrough in the technology of energy storage.
But as you said, there are still a lot of limitations concerning the possibility of commercializing its usage. Hopefully, something would be discovered in the nearest future.
Nice post
Very educative and informative posts. Science and technology drive any viabke economy. Thanks for the post I enjoyed reading
thank you for information