A couple of weeks ago, when I got behind the wheel of the car and tried to start it, I had the unpleasant experience of checking that the battery was discharged. After proceeding for a few hours to recharge it with a car battery charger, it occurred to me that this could be a good topic to explain how the battery or accumulator of lead that all vehicles have and that provides them with the electric current necessary for the starter motor, spark plugs, lights, etc. When the vehicle is running, the electric current that is produced in the dynamo goes through the accumulator or battery and regenerates the reagents.

Source
An accumulator does not stop being a special type of battery that can be recharged repeatedly. The main aspect that allows a system to be rechargeable is the fact that the products originated in the discharge reaction is deposited on the electrodes. Therefore, if an electric current is passed through it, the original substances can be regenerated.
The lead accumulator was invented by Gastón Planté in 1859 and has been incorporated into all cars with small variations.

Source
The anode is formed by a group of metallic lead sheets in the shape of a grid, with the spaces filled with fluffy gray lead. The cathode is composed of another group of lead sheets in the form of a grid but filled with lead dioxide (PbO2). The sheets that form the anode and those that form the cathode are interspersed inside the battery and bathed in a solution of aqueous sulfuric acid (H2SO4) that acts as an electrolyte.
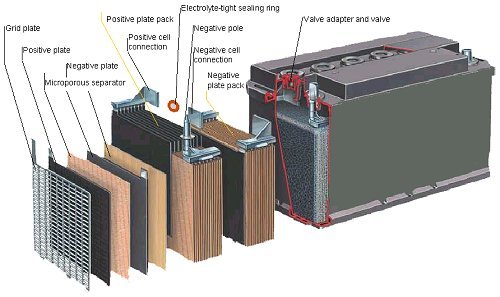
Source
Each accumulator produces a potential difference of 2.1 volts, and as commercial batteries are composed of 6 pairs of sheets, the total voltage of the battery will be about 12.6 volts.
When the battery produces electrical current, the electrochemical reactions are:
Anode:Pb + SO42- ⇌ PbSO4+2e-(pole -)
Cathode: PbO2+4H++SO42-+ 2e-⇌ PbSO4+ 2H2O(pole+)
Pb(s)+PbO2(s) + 2H2SO4(aq) ⇌ 2PbSO4(s) + 2H4O(l)

Source
As we can see, plumbous sulfate (PbSO4) is formed at both the anode and the cathode. When DC current passes through the battery, and in the opposite direction to that supplied by it, the reactions of the electrodes are reversed and their capacity to produce electrical energy is regenerated. The overall reaction is exactly the reverse reaction of the discharge:
DOWNLOAD → ← LOAD
Pb(s)+PbO2(s) + 2H2SO4(aq) ⇌ 2PbSO4(s) + 2H4O(l)
The performance of a battery oscillates between 94 and 98%, storing a load between 3.6 x 10 4 and 7.2 x 104 Coulombs, according to the size of the plates. If a battery is left unloaded for a prolonged period of time, the PbSO4 crystals that form on the electrodes are so large and can not be converted into Pb and PbO2 by the passage of electrical current.
These PbSO4 crystals each charge/discharge cycle become increasingly larger and prevent the anode and cathode reactions from being carried out completely, lowering the battery performance. It is said then that it has been sulfated. When this happens, it is necessary to replace the battery with a new one.
The state of a lead accumulator can be controlled by measuring the density of the H2SO4 solution. This is what they do in the mechanical workshops with their "specific gravity tester". In a fully charged battery, the concentration and density of the H2SO4 solution are high; when it is discharged the solution is more diluted and therefore of lower density.

Source
So, we see how an invention developed in the mid-nineteenth century continues to make life easier, providing us with an adequate voltage to start the engine of our cars.
For more information visit:
- https://www.batterystuff.com/kb/articles/battery-articles/secret-workings-of-a-lead-acid-battery.html
- https://alchetron.com/Gaston-Plant%C3%A9
- http://www.internetdict.com/ms/answers/how-battery-works.html
- https://en.wikipedia.org/wiki/Lead%E2%80%93acid_battery
- https://www.progressivedyn.com/service/battery-basics/
I invite you to comment on this topic, I await your comments.
Something to note is that most vehicles made past 1960 use an alternator instead of a dynamo as it is cheaper and lasts longer, the brushes in a commutator wear down really fast. After this they will tend to pass through a transformer to first fix the voltage and then they will pass through a full-bridge rectifier.
An accumulator is just something that accumulates energy, not necessarily a battery. For instance, a capacitor is also an accumulator and so it a fly wheel.
There is a stem-espanol that you can write in spanish and get the steemstem upvote. At multiple points there are sentences that are difficult to understand exactly what you are meaning and may have been cause of translation error. If you are more comfortable with writing in Spanish then please do so! Especially since your references aren't English.
Thank you very much for the advice and clarification regarding my publication, I will take into account in publishing in Spanish and thus also contribute to the steemstem community in Spanish, I hope the support will continue on your behalf. Thank you very much.