Nanotechnology: poison and toxicity
source

In spite of how difficult it can be to recognize it, scientists working in certain specific disciplines strive to generate new applications of their study materials (nanomaterials, genes, etc.) without adequately assessing their impact on the environment and public health. Worse still, they often venture to defend their goodness, hiding the collateral problems they can generate. And it is not about some isolated colleagues, but a good part of the scientific community involved. Therefore, nanomaterials are already used in many products that are eventually discarded in the environment, either water or soil.
The indiscriminate use of nanoparticles is alarming since it has just been demonstrated that they accumulate in living beings as they ascend along the food chain, showing serious toxic effects. We are talking about bioaccumulation or biomagnification. The environmental problem can have dramatic consequences if the necessary measures are not adopted urgently since its use extends to a multitude of products of all kinds. For the time being, we already know that the soils and waters (and the biota that they harbor) are reservoirs of these small particles, which do not seem to be precisely benefactors, at least in many cases.
source
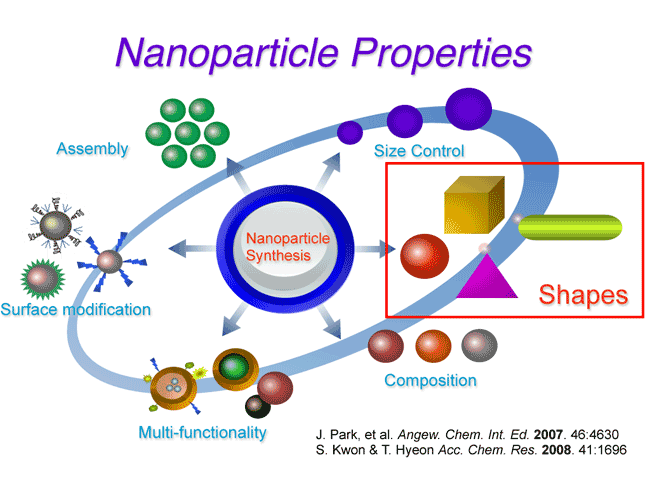
Potential risks
In the short term, critics of nanotechnology point out that there is a possible toxicity in new classes of nanosheets that could negatively affect the stability of cell membranes or distort the immune system when inhaled or ingested. An objective assessment of risks can take advantage of the amount of experience accumulated with well-known microscopic materials, such as soot or asbestos fibers.
There is a possibility that nanoparticles in drinking water may be harmful to humans and other animals. It has been discovered that colored cells exposed to titanium dioxide particles decompose at a faster rate than normal. Titanium dioxide nanoparticles are commonly used in solar screens, making them transparent, unlike large particles of titanium dioxide, which make the sunscreen appear white.
source

The environmental gains derived from the use of nanomaterials could be lost in part, due to the processes necessary for their manufacture; This is what the University of Illinois at Chicago says, they claim strict requirements on the purity of materials, the lowest tolerances for defects and the production processes with returns in many other economic sectors.
Until now, more attention has been paid to the possible toxic effects derived from exposure to nanoparticles. But the polluting and energetic considerations of the technologies used for their smaller production also need attention, can cause greater environmental burdens than the processes associated with conventional manufacturing.
Promises to reduce waste, clean industrial pollution, provide clean water and improve the efficiency of production and use of energy.
Two separate studies that were published in 2008 found that certain carbon nanotubes cause a pathogenicity similar to that of asbestos and the appearance of mesothelioma in laboratory rats.
source
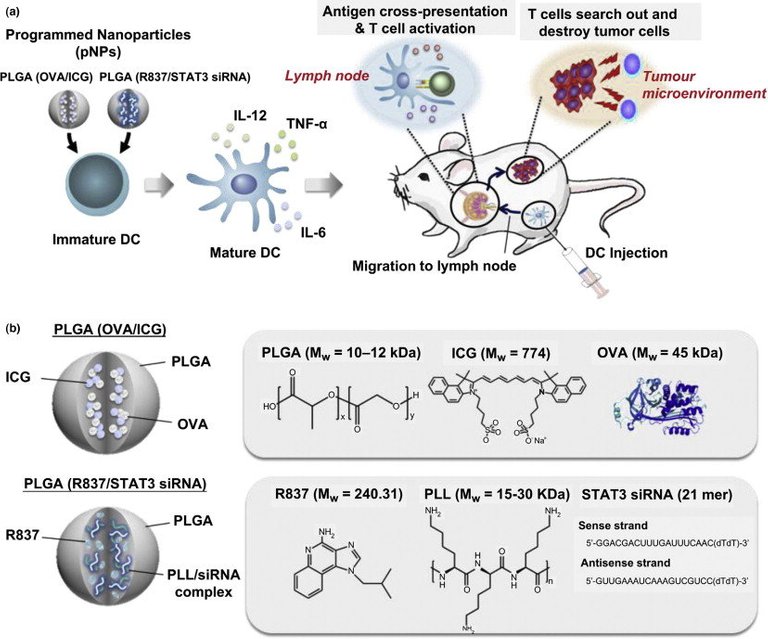
- Studies in vitro (in test tubes) and in vivo (with animals) have shown that the manufactured nanoparticles, which currently have a wide commercial use, such as zinc, zinc oxide, silver and titanium dioxide, pose new risks of toxicity. Nanoparticles could also cause long-term pathologies.
source
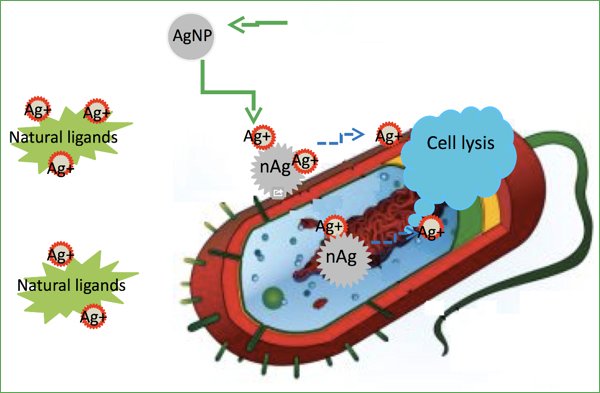
Use of carbon nanotubes to eliminate lead in water and tests on other metals.
There are several concerns about nanomaterials in the environment, including: Toxicities of particles and fibers of nanomaterials. The fate of the contaminating material by adsorption of water.
Biodegradability and persistence of the environment. The effectiveness of methods to eliminate toxic nanomaterials from the environment. Improper use of nanomaterials.
Nanoparticles of titanium dioxide (TiO2 and carbon nanotubes that act with contaminants (organic and inorganic) in the water for the purpose of adsorption and aggregation.