INTRODUCTION
1.1 Background information
Plankton are a diverse group of organisms that live in the water column of water bodies and that cannot swim against water current. They provide a crucial source of food to many large aquatic organisms, such as fish and whales. Drifting aquatic microorganisms are collectively termed phytoplankton if they are of plant origin and zooplankton if they are of animal origin (Tebutt, 1983).
“Zooplankton” is derived from the Greek zoon meaning “animal” and planktos meaning “wanderer” or “drifter”. Zooplankton is a categorization spanning a range of organism sizes including small protozoans and metazoans. It includes holoplanktonic organisms (diatoms, radiolarians, dinoflagellates, formaninifera, amphipods, krill, copepods, salps, etc.) whose life cycle lies within the plankton, as well as meroplanktonic organisms that spend part of their lives in plankton before graduating to either the nekton or a benthic sessile existence. Although, zooplankton is primarily transported by ambient water currents, many have locomotion, used to avoid predators (as in diel vertical migration) or to increase prey counter rate.
Rivers, lakes, and reservoirs are considered favourable environment for zooplankton communities which may establish diverse assemblages in relatively short periods of time after impoundment (Rocha et al., 1999). Just as some species of organisms can be limited within a geographical region, so is zooplankton. However, species of zooplankton are not dispersed uniformly or randomly within a region of the ocean. Instead, patches of zooplankton species exist throughout the ocean. Though few physical barriers exist above the mesopelagic, specific species of zooplankton are strictly restricted by salinity and temperature gradients; while other species can withstand wide temperature and salinity gradients. Zooplankton patchiness can also be affected by biological factors, as well as other physical factors. Biological factors include breeding, predation, concentration of phytoplankton, and vertical migration. The physical factor that influences zooplankton distribution the most is the mixing of the water column (upwelling and downwelling along the coast in the open ocean) that affects nutrient availability and in turn, phytoplankton production. The assemblages of zooplankton often differs in diversity and abundance from water body to water body, from location to location within each water body, from geological region and also with time (intra annual between years) and are structured by fish predation, competition, aquatic macrophytes (Jackson and Schmitz, 1987), and physical, chemical, and biological factors (Sampio et al., 2002).
Zooplankton are classified by size and/or by developmental stage. Size categories include:
⦁ Picoplankton (< 2 µm)
⦁ Nanoplankton (2- 20 µm).
⦁ Microplankton (20-200 µm).
⦁ Mesoplankton (0.2 – 20 mm).
⦁ Macroplankton (20- 200 mm).
⦁ Megaplankton (200 mm).
1.2 Importance of zooplankton
Zooplankton occupy an important trophic level in the aquatic ecosystem as they constitute the most important link in energy transfer between phytoplankton and higher aquatic fauna (Ileoba, 2002).
Zooplankton play an important role in a lake’s ecosystem and food chain. Zooplankton have vital roles in lake food webs because they regulate the populations of phytoplankton by consuming them (Wetzel 1983). Unlike algae, or phytoplankton, they cannot produce their own food. They are responsible for eating millions of little algae that may otherwise grow to an out-of-control state. In fact, as mostly filter feeders, a community of zooplankton can filter through the volume of an entire lake in a matter of days. However, not all algae are edible and often times it’s the blue green algae that we would like to see disappear that can’t be eaten.
Zooplankton are also a valuable food source for planktivorous fish and other organisms. The presence or absence of healthy zooplankton populations can determine some commercial fish success in both freshwater and saltwater bodies. By ensuring that the lower parts of the food chain are healthy, we can protect the higher ordered organisms such as fish, whales and even humans.
Furthermore, zooplankton are so closely linked to the environment, and since they tend to respond to changes more rapidly than the larger aquatic animals such as fish, these microorganisms have proved to be a valuable indicator of apparent and subtle alteration in the quality of the freshwater ecosystem (Gibbon and Funk, 1982). However, they indicate the effect of low levels of chemical pollution in water body and because of their more important role in the food chain and energy flow, they are a good indicator of pollution in biological monitoring (Rutherford et al., 1999; Soberon et al., 2000).
Changes in zooplankton composition (diversity and density) over a long period of time have also been shown to be due to their responses to river ageing. For instance the dominance of Cyclops vernalis and Bosmina sp in the Pawnee reservoir Lincoln (USA) indicates that the these two zooplankton were tolerant to the changes in the physical conditions resulting from reservoir ageing and biotic interactions in the reservoir for a long period of time (Popp et al., 1996). Some copepod zooplankters are known to be intermediate hosts for many helmiths and nematodes as well as playing an important role in the spreading of cholera. For instance, cholera outbreaks in developing countries have been linked to zooplankton that support the growth of Vibrio cholerae, the infectious agent of cholera, and can aid in its spread through unfiltered or poorly filtered drinking water (Huq et al., 1996). It has been observed that cholera outbreaks are rampant after plankton blooms (Huq et al., 1996).
1.3 Objectives of study
⦁ To investigate the Taxonomic composition, and occurrence of zooplankton species from Ifewara Reservoir.
⦁ To determine the variation in the abundance of zooplankton across the different selected sampling locations from Ifewara Reservoir.
CHAPTER 2
LITERATURE REVIEW
2.1 Taxonomic composition of Zooplankton
The zooplankton of inland waters derives its name mainly from the protozoa, the rotatoria (rotifers), the cladocera, and the copepod. In addition, there are occasional minor elements contributed ostracod crustaceans, water mites (arachnids), larval mollusks, mysid crustaceans and the larvae of the insect Chaoborus (Cole, 1975).
Seasonal distributions of zooplankton are difficult to generalize in some regions. Variations in seasonal distribution commonly exist, and furthermore, little is known about seasonal succession in warm regions (Wetzel, 1983). This study would provide information on the zooplankton population distribution in the Ifewara Reservoir.
2.1.1 Rotifers
An animal like a large maggot which could contract itself into a spherical figure and then stretch itself out again; the end of its tail appeared with a forceps-like that of an earwig (Harris, 1696).
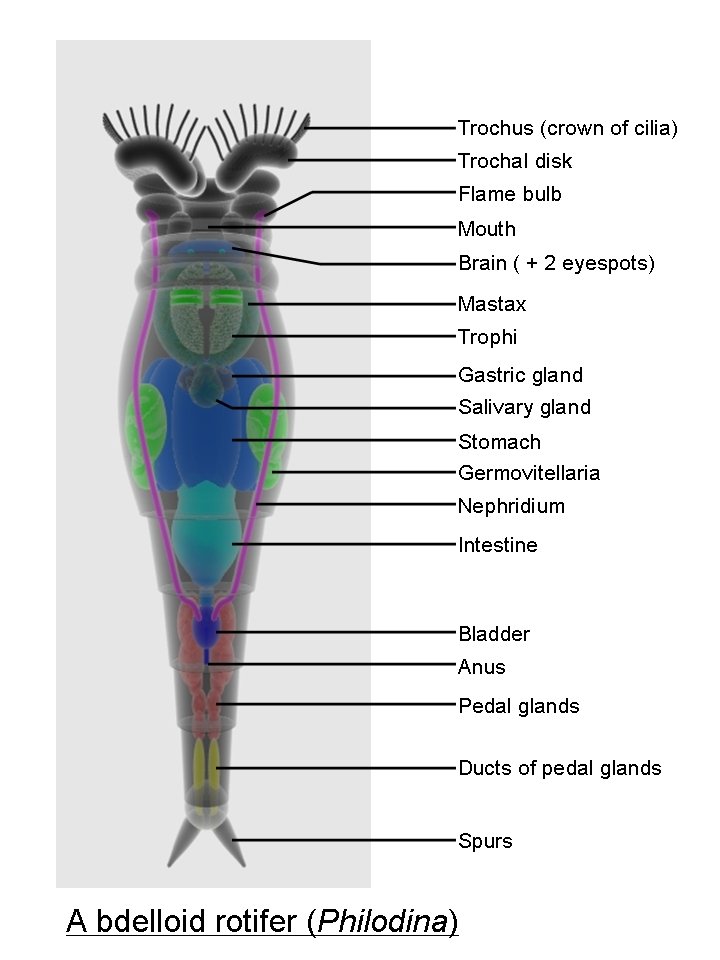
Rotifers vary widely in their morphology, but most species have a distinguishable head, trunk, and foot regions as well as an elongated body (Wetzel, 1983), as seen in Fig 2.1. Feeding occurs by moving the organic matter to the mouth cavity by using cilia (Wetzel, 1983). This ciliated region around the mouth, called a corona, is also used for locomotion. All rotifers have a muscular pharynx, the mastax, which contains a set of jaws called trophi (Wallace and Snell, 2010).
Conochilus, another species that will be studied, can exist alone or as a colony. Colonial rotifers usually form colonies of only a few individuals or of intermediate sizes. The Larger colonial formation may be an adaptive response to heavy predation (Walsh et al., 2006). Conochilus form colonies autorecruitively in which the young join their parental colony, increasing the size of the colony (Wallace and Snell, 2010). Consequently, genetic relatedness is high in autorecruitive colony formation, and possible consequences include a higher vulnerability to parasitic infections and a reduced genetic diversity among diapausing embryos (Wallace and Snell 2010).
However, colonial forms display a longer lifespan and an enhanced ability to avoid engulfment by predators than solitary individuals (Walsh et al., 2006; Wallace and Snell, 2010).
Rotifers have widely served as biological indicators and in previous studies, the relationship between toxins and rotifer predator-prey interactions and composition has been explored (Lagadic and Caquet, 1998; Wallace and Snell, 2010). Such toxicity tests included exposing the rotifers to insecticides, crude oil, heavy metals, and petrochemicals (Radix et al., 2000). Previous studies have found rotifers to be good indicators of water quality (Lagadic and Caquet, 1998; Wallace and Snell, 2010).
2.1.2 Cladocera
Crustacean arthropods are largely aquatic, and in freshwater systems, cladocerans and copepods are the most prevalent of the crustacea. Physical characteristics include jointed appendages and a segmented body, but this noticeable segmentation has been lost in the cladocerans (Wetzel, 1983). Cladocerans, commonly known as “water fleas,” have a distinct head, a single compound eye and a large mandible for grinding food particles (Wetzel, 1983) as seen I Fig 2.2.

Fig 2.2 Diagram of cladoceran
Cladocerans reproduce mostly asexually via parthenogenesis but can reproduce sexually based on the environmental conditions (Zadereev, 2003). Resting eggs from fertilization or, in some species, asexual reproduction can be produced if the presence of crowding or toxic food is signaled (Dodson et al., 2010, Zadereev, 2003). These eggs are resistant to desiccation and can survive on dry land or in water sediments for lengthy periods of time (Mort, 1991). Leaving diapause, which is a halt in its growth cycle, requires favorable stimuli from the environment (Dodson et al., 2010). Development in size occurs through molting. The rate at which they develop depends on the amount of stored energy. Cladocerans feed on algae, small rotifers, and copepod nauplii (Dodson et al., 2010). Their metabolic rate is variable with temperature, and death can occur above the required optimal temperature (Dodson et al., 2010).
Under food limiting conditions, a smaller body size is favored (Dodson et al., 2010). For example, Bosmina may be able to out-compete a larger species because it could grow faster when food is limited (Sommer et al., 1986). Additionally, cladocerans that have a larger body size seem to be scarce when fish are present as fish are visual predators (Sommer et al., 1986). Cladoceran populations expand in conjunction with algae bloom (Abrantes et al., 2006). They are most abundant in the spring in northern areas and in the rainy season in southern areas. Growth is limited during cold conditions (Abrantes et al., 2006, Dodson et al. 2010). Daphnia and, to a lesser extent, Bosmina can effectively graze on large abundances of algae, and therefore, contribute to improving water quality, which is dependent on algae dynamics (Dodson et al., 2010).
2.1.3 Copepoda
Copepods have a segmented body with an exoskeleton and five pairs of jointed appendages (Reid and Williamson, 2010). The first antennae, one of the notable appendages, have roles in reproduction, locomotion, and feeding. Calanoids and cyclopoids can be distinguished by their first antennae, with calanoids possessing the longer antennae (Wetzel, 1983). Copepods live in a variety of aquatic habitats, such as marine and freshwater environments. They feed on algae, other invertebrates, and larval fish. Cyclopoids grasp their prey and eat larger prey than calanoids, which create currents to bring in food (Reid and Williamson, 2010). The free-living copepods are divided into three suborders: the Calanoida, the Cyclopoida, and the Harpacticoida (Wetzel, 1983). The relative abundances of calanoids versus cyclopoids can change based on food availability. When productivity increases, calanoids decrease in abundance while cyclopoids and cladocerans increase in abundance (Soto and Hurlbert, 1991).
Calanoids may survive on lower densities of food than cyclopoids, which may contribute to this difference (Reid and Williamson, 2010). Unlike cladocerans and rotifers, copepods only reproduce sexually and have a larval stage called the nauplius. Temperature, food availability, and predation heavily influence their mating behavior and variations in their dynamics (Reid and Williamson, 2010; Varpe et al., 2007). Egg development and clutch size have been known to be dependent on temperature for copepods (Devreker et al., 2009). They are also known to have a broad adaptation to unfavorable environmental conditions. They respond by reducing their metabolic rate and entering diapause (Reid and Williamson, 2010). Copepods comprise most of the biomass and productivity of freshwater environments (Reid and Williamson, 2010). Since they are omnivorous, they are important in aquatic food chains because they can regulate both algae and zooplankton populations (Reid and Williamson, 2010). Predatory copepods can impose high pressure on their prey and can inhibit the growth of other invertebrates, such as smaller rotifer species (Brandl, 2005).
Copepods also support other copepod and fish populations (Reid and Williamson, 2010). However, copepods do serve as intermediate hosts for parasites, such as flukes and tapeworms. Their exoskeleton acts as a reservoir for pathogenic bacteria including Vibrio cholerae (Huq et al., 1996) and Enterococcus faecalis. If the infected copepod is consumed in the drinking water, serious illnesses can occur in humans (Reid and Williamson, 2010).
References
Abrantes, N., Antunes, S.C., Pereira, M.J., Goncalves. F. (2006). Seasonal succession of cladocerans and phytoplankton and their interactions in a shallow eutrophic lake (Lake Vela, Portugal). Acta Oecologica 29:54-64..
Aleskeev, V.R. (2002). Copepoda. In: Fernando C. H. (.ed). A guide to Tropical Freshwater zooplankton; Identification, Ecology and Impact on Fishes. Backhuys Publishers, Leiden, The Netherlands. Pp 123-187.
Saint-Jean, L. (1983). The zooplankton. In J.P. Carmouze, J.R. Durand and C. Leveque (eds), Lake Chad: ecology and productivity of a shallow tropical ecosystem. Nigeria. p.199- 232.
Sampio, E. V., Rocha, O., Matsunmura-Tundisi, T. and Tundisi, J.G (2002). Composition and abundance of zooplankton in the limnetic zone of seven reservoirs of the paranapanema Rivers, Brazil J. Biol. 62: 525-545.
Soberon, J., Rodriguez, P., and Vazguez-Dominguez. (2000). Implications of the hierarchical structure of biodiversity for the development of ecological indicators of sustainable use. Ambio 29 (3): 136-142.
Source
Copying/Pasting full or partial texts without adding anything original is frowned upon by the community. Repeated copy/paste posts could be considered spam. Spam is discouraged by the community, and may result in action from the cheetah bot.
More information and tips on sharing content.
If you believe this comment is in error, please contact us in #disputes on Discord
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://smartech.gatech.edu/bitstream/handle/1853/36553/Thesis.pdf