Introduction
Malaria as a disease has been documented for thousands of years, throughout Chinese, Mesopotamian, Egyptian, Hindu, Greek, and Roman history (Cox, 2010). Its name, which likely comes from the Italian mala aria, refers to the old belief that it is caused by “bad air”. In 1880, Charles Louis Alphonse discovered one of the Plasmodium parasites responsible for malaria, which was followed by the findings that mosquitos are responsible for transmitting it when feeding on humans (Cox, 2010).
Nowadays, there are over a hundred known species of the malaria parasite, six of which are known to infect humans: P. falciparum, P. vivax, P. ovale wallickeri, P.ovale curtisi, P. malariae, and P. knowlesi (Milner, 2018). Among those, P. falciparium and P. vivax are those most common, with P. falciparum being responsible for about 99.7% of malaria cases in the World Health Organization (WHO) African Region in 2018 (“World malaria report 2019,” 2019).
The symptoms of malaria infection are similar to those of flu, with fever, headaches, vomiting, and fatigue. Their occurrence tends to be cyclic and correlates with the life cycle of Plasmodium. Untreated, malaria infection can lead to cerebral malaria, which affects the brain and can cause brain damage, coma, and other complications. Malaria infections especially endanger pregnant women and young children. During pregnancy, malaria can severely impact the unborn child, potentially leading to stillbirth or miscarriage. (“Malaria - Symptoms - NHS,” 2018).
As mentioned above, the life cycle of Plasmodium tends to have a cyclic pattern. It is complex and varies between different Plasmodium species, but they all infect red blood cells and grow inside them, destroying the cells in the process (Tuteja, 2007).
Because malaria is such a dangerous disease, many countries have tried to get rid of it as soon as they developed techniques to do so. With the help of changes in land use, insecticides and the control of mosquitos carrying the parasite, malaria has been successfully eliminated from the US and most of Europe early in the twentieth century. Similar efforts have been made on a global scale in the 1950s/60s using the insecticide DDT, which has been successful in India, Sri Lanka, and the former Soviet Union. However, the mosquitos began developing resistance to DDT, making it less useful and too costly. On top of that, there was a lack of action in sub-Saharan Africa, leaving a large area open to the parasite (Greenwood and Mutabingwa, 2002).
In 1998, the WHO, United Nations Development Program, World Bank, and UNICEF founded the Roll Back Malaria (RBM) partnership. Their goal was to bring worldwide malaria deaths to zero, or at least almost zero, by 2015 and maintain these levels to eventually fully eradicate malaria (“Roll Back Malaria Partnership,” 2015).
Despite their efforts and multiple organisations and businesses joining them, this goal is still out of reach. In 1995, almost 1 million deaths occurred in sub-Saharan Africa due to malaria (Snow et al., 1999). In 2018, that number shrunk to 405 000 deaths worldwide, although most of them (94%) still occurred in the WHO African region, allowing these numbers to be roughly comparable (“World malaria report 2019,” 2019). Figure 1 illustrates the huge reduction in the mortality rate after the year 2000.
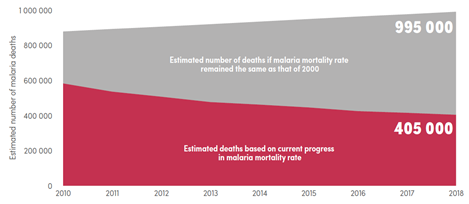
Figure 1 Comparison of current estimated malaria deaths with expected deaths had malaria incidence remained at 2000 levels globally. Source: WHO World Malaria Report 2019
And while a reduction of mortality by over 50% can be considered positive progress, these numbers mean that there are still hundreds of thousands of people dying from malaria each year – mainly children under five years old, according to the WHO. This is a loss of life that should be possible to prevent.
Why is this the case, why are people still dying from malaria?
As it is at the moment, three primary reasons can be considered the root causes: Increasing drug resistance, the spread of malaria species through human migration, and lack of access due to poverty or other structural injustices.
The following essay will discuss the impact and underlying mechanisms of the three root causes.
Discussion
Drug resistance on the rise
For recovery from malaria to be possible, patients need to be treated with the appropriate medication. If this medication loses its effectiveness, there is not much that can be done for people with the disease, and the consequence can be a worsening of the symptoms and eventually death.
Since its introduction in 1934, the drug chloroquine has been used to treat malaria. It inhibits the feeding process of Plasmodium inside human red blood cells, keeping it from growing and eventually causing its death (Yayon et al., 1984). A common problem with widely used drugs, however, is that the targeted pathogens tend to slowly develop a resistance against them – which has become the case for chloroquine soon after its introduction.
Already back in 1961, chloroquine resistance has been reported in Colombia with P. falciparum either barely reacting or even not at all to usually effective treatment with the drug (Young and Moore, 1961). More cases followed, in 1989, chloroquine resistance of P. vivax has been observed in two soldiers returning from Papua New Guinea. They had both taken prophylactic treatment, which had proven unsuccessful as they caught the disease regardless. To treat the malaria infection, a high dose of chloroquine had been administered but again without effect (Rieckmann et al., 1989). Later, in 1998, 23% of treated P. vivax cases in transmigrants in Indonesia exhibited chloroquine resistance (Baird et al., 1998). The most critical part about this development wasn’t the resistance itself, but the fact that the two most common Plasmodium species both developed it. Multiple species being resistant results in an overall weakening of the effectivity of chloroquine.
Even today, the chloroquine resistance keeps spreading, with resistant P. vivax appearing in Brazil, Bolivia, and French Guiana (Musset et al., 2019). Among the reasons for this phenomenon is not just the natural adaption of Plasmodium to the selective pressure, but also the administration of too low dosages and even low-quality drugs (Newton et al., 2016). Both factors allow the parasite to evolve faster while at the same time causing people to receive insufficient treatment, possibly resulting in a persisting infection.
One possibility to treat malaria parasites that have developed resistance to one drug is to combine three different antimalarial drugs, making it less likely for Plasmodium to have a defence mechanism against them. Some risks that go along with this approach are increased side effects and costs, making it an effective but not ideal approach (Boni et al., 2016).
To make the situation more dire, chloroquine is not the only drug that the different Plasmodium species have developed a resistance against so far. There are documented cases for all currently available malaria medications, most recently artemisinin-based combination therapy (Thu et al., 2017). Artemisinin can be considered chloroquine’s successor, as its global use is increasing. Despite the emerging resistance, triple artemisinin-based combination therapies are being developed, but to properly design them a better understanding of the resistance mechanisms is required (Haldar et al., 2018). If a resistance mechanism is known, it can be avoided more easily.
With artemisinin and other drug resistances in P. falciparum becoming an increasing problem, it is necessary to develop new, more effective antimalarial drugs. If that is not possible, malaria has to be eradicated faster than resistance can develop and spread (Menard and Dondorp, 2017). As long as neither the discovery of new drugs nor the eradication of malaria are achieved, the spread of resistance needs to be contained as well as possible, which leads to the question of migration.
Migrating populations and national crises
The global elimination of malaria is a stepwise process, progressing country by country. While most countries that have officially eliminated malaria stay free of the disease, there are occasional reintroductions that threaten the disease-free status and the overall goal of eradication (Emms et al., 2018). As mentioned in the previous section, a high portion of resistant P. vivax strains has been documented in transmigrants, which points to another problem: Migration.
There is evidence for the female disease-carrying mosquitos travelling long distances and spreading Plasmodium through that (Huestis et al., 2019). Still, this behaviour is not the key type of migration happening. The movement of human populations contributes to the spread and reintroduction of malaria to a significant degree.
In border regions of countries at risk, the prevalence of malaria is higher than in other areas and correlates with people frequently moving across borders (Wangdi et al., 2015). In addition to the increased prevalence, it becomes harder for the countries to contain and eliminate malaria outbreaks in their region, especially if the neighbouring country allocates fewer resources towards this cause.
An example of migrants introducing a higher burden of disease to their country of destination was documented on the Thai-Myanmar border in 2017. At that point in time, most of the P. falciparum infections were observed in recent migrants to Thailand, which tends to have a lower malaria incidence rate than Myanmar (Sriwichai et al., 2017).
But human migration carries another risk factor beyond spread and reintroduction: The opportunity for Plasmodium species to increase their genetic variety and further develop resistance – and possibly pathogenicity. P. vivax has already been shown to be profiting from this migration-induced great genetic variety (Lo et al., 2017) and is likely not the only Plasmodium species experiencing that, which adds to the previously discussed drug resistance.
Beyond “normal” migration patterns due to availability of work or even tourism, wars and crises can force even larger migration between countries. A very recent example of this phenomenon is Venezuela. The economic and social crisis has destabilised the country in multiple ways. Shortage of antimalarial drugs and lacking control efforts from the government enable the spread of malaria, and the affected people are difficult to contain. There have been reports of imported malaria cases from Venezuela, even on the Ecuador-Peru border region (Jaramillo-Ochoa et al., 2019). Of course, people aren’t leaving specifically because of malaria; the disease is unlikely to be a major consideration for most of those leaving. Food insecurity and hyperinflation (Doocy et al., 2019) are much more of an influence, driving often illegal mass emigration to other countries on the South American continent (Hanson, 2018).
For those who stay in Venezuela instead of fleeing to neighbouring countries, the breakdown of their economy and instability of their government is accompanied by the outbreak of several other diseases no longer properly addressed by the crumbling healthcare system (Rodríguez-Morales et al., 2019). Even vaccine-preventable diseases are coming back (Paniz-Mondolfi et al., 2019). Both factors add to the pressure on the Venezuelan population.
What the example of Venezuela demonstrates is that a country in which the population suffers from a higher burden of disease also tends to provide less support to the suffering community, as these things often go hand-in-hand. People who need access to healthcare and preventative methods most are frequently those who are left behind.
Lack of access among those who need it most
The treatment and prevention of malaria are associated with a certain cost, not just for the people receiving the care but also for the government in their country. The problem of very-low-income countries being unable to afford necessary interventions, even if these interventions are technically cost-effective, is an old one (Goodman et al., 1999).
In Ghana, a lower-middle-income country, the average monthly net income of a worker in the banana industry in 2017 was about US$152 (Smith et al., 2017). Considering that treating an under-five child with malaria in Ghana costs a family on average US$4.91 (Dalaba et al., 2018), treatment might not be a viable option for everyone, especially families with an even lower income – or from even poorer countries with a healthcare system that carries less of the incurring cost.
And although there are worldwide investments by richer countries and companies into malaria relief with global spending on malaria rising 10-fold between 2000 and 2008 (Cowman et al., 2016), the numbers have been falling again in more recent years (figure 2). While in 2017 US$3.2 billion had been available, only US$2.7 billion have been in 2018 (“World malaria report 2019,” 2019). If that trend keeps dropping, it will severely negatively impact the progress made so far.
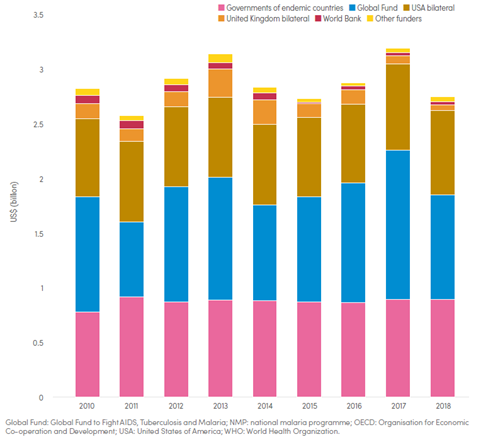
Figure 2 Funding for malaria control and elimination 2010-2018, by channel. Source: WHO World Malaria Report 2019
Beyond the cost of treatment, malaria prevention, which should be the preferred focus, is also associated with the need for resources. In poorer and more rural regions, protections like insecticide-treated nets (ITNs) are not always accessible, and less complicated measures like closing windows early in the evening have to be done regularly, or they are ineffective (Musoke et al., 2016). In Malawi, people do not always sleep in their beds under the safety of already owned ITNs because of problems with bed bugs (Parker, 2018), which shows how even seemingly unrelated problems can affect anti-malaria measures.
Of course, knowing how to protect oneself most effectively against malaria is as crucial as having access to the methods, but poor and rural regions are at a disadvantage here too. Populations that do not receive information through billboards, radio announcements, community events or other methods are considerably less likely to protect themselves with ITNs or by taking prophylactic medication (Yaya et al., 2018). According to the WHO World Malaria Report, only about 50% of the sub-Saharan population slept under an ITN (figure 3).
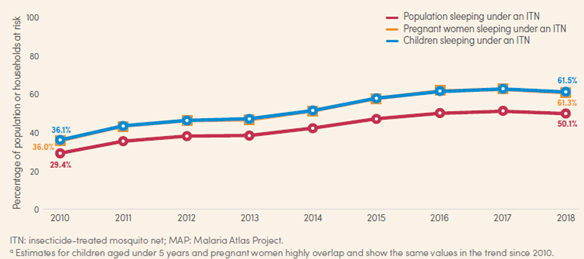
Figure 3 Percentage of population at risk, pregnant women and children aged under 5 years sleeping under an ITN, sub-Saharan Africa, 2010-2018. Source: WHO World Malaria Report 2018
Especially first-time mothers and illiterate pregnant women are less likely to use ITNs because they either do not know about them, do not have access, or cannot afford them (Bhalla et al., 2019). And if an HIV infection is also present, which tends to be common in African countries that have a high malaria burden, prophylactic treatment with antimalarial drugs might not even be an option. Concomitant cotrimoxazole prophylaxis (CTXp) is widely recommended to HIV-positive pregnant women to avoid opportunistic infections, but it is believed to have serious drug interactions with the sulfadoxine-pyrimethamine given as prophylaxis. As a result, pregnant women, who are already a vulnerable group, are even more likely to contract malaria. If prevention is difficult, treatment is usually not easier, particularly because the infection itself already brings the risk of miscarriage or stillbirth.
With lack of information, availability, and access arguably being the biggest and deadliest of the three discussed reasons it comes to no surprise that the highest incidence and mortality rate of malaria occurs in poor countries with predominantly rural populations, specifically in Africa (“World malaria report 2019,” 2019).
Conclusion
As it is the case for most global health problems, there is no single reason why malaria is still not eradicated and why people are still dying from it despite great efforts being taken against both. The resistance of Plasmodium against anti-malarial drugs, migration of human populations that keep spreading malaria between countries, and a lack of access to interventions especially in poor countries are barriers that need to be overcome to push the mortality rate of malaria to zero.
To address the increasing resistance against a multitude of drugs, pharmaceutical companies and other research facilities will need to focus more on finding new ways to treat a malaria infection and find reasons why current resistances exist. A reversal of resistance against chloroquine has already been shown to be possible in monkeys (Obaldia et al., 2018), opening up the possibility to keep using old drugs, if new ones cannot be developed. One factor that makes it more unlikely for companies to focus on this research is the fact that most malaria cases nowadays occur in poor regions. People living there won’t be able to pay as much for the drugs as richer countries, possibly making it a bad investment.
The spread and mutation of malaria through human migration could pose a larger threat in the future, as wars and climate change force more people to leave their home countries. Climate change itself also opens up more countries as a viable breeding ground for malaria, that would have otherwise been too cold, making the movement of large populations even more of a risk. To ensure malaria-free countries keep their status, steps must be taken to stop the spread, although that might not be possible in all situations.
Having said that, the biggest factor for the persistence of malaria and deaths caused by it is a lack of access to prophylactic protection and treatment. The reason for this lack of access is usually of structural nature: Countries being poor and unable to support their inhabitants, or persisting instability inside a country due to wars, economic crisis, or the aftereffects of colonialism. All of these reasons are not just affecting malaria but also the overall health of people. Even if drug resistance is overcome and migration between countries, or at least the associated spread of malaria, stops, people will keep dying of malaria if this structural injustice is not addressed.
If people are to stop dying from malaria, more funding and more focused efforts are needed to not only tackle malaria itself but primarily the circumstances that keep people from using available protections and medications.
Bibliography
Baird, J.K., Fryauff, D.J., Richie, T.L., Masbar, S., Kain, K.C., Tuti, S., Patipelohi, R., Bangs, M.J., Leksana, B., Mardi, A., 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59, 513–518. https://doi.org/10.4269/ajtmh.1998.59.513
Bhalla, D., Cleenewerck, L., Okorafor Kalu, S., Abubakar Gulma, K., 2019. Malaria Prevention Measures among Pregnant Women: A Population-Based Survey in Nnewi, Nigeria [WWW Document]. Sci. World J. https://doi.org/10.1155/2019/6402947
Boni, M.F., White, N.J., Baird, J.K., 2016. The Community As the Patient in Malaria-Endemic Areas: Preempting Drug Resistance with Multiple First-Line Therapies. PLOS Med. 13, e1001984. https://doi.org/10.1371/journal.pmed.1001984
Cowman, A.F., Healer, J., Marapana, D., Marsh, K., 2016. Malaria: Biology and Disease. Cell 167, 610–624. https://doi.org/10.1016/j.cell.2016.07.055
Cox, F.E., 2010. History of the discovery of the malaria parasites and their vectors. Parasit. Vectors 3, 5. https://doi.org/10.1186/1756-3305-3-5
Dalaba, M.A., Welaga, P., Oduro, A., Danchaka, L.L., Matsubara, C., 2018. Cost of malaria treatment and health seeking behaviour of children under-five years in the Upper West Region of Ghana. PloS One 13, e0195533. https://doi.org/10.1371/journal.pone.0195533
Doocy, S., Ververs, M.-T., Spiegel, P., Beyrer, C., 2019. The food security and nutrition crisis in Venezuela. Soc. Sci. Med. 226, 63–68. https://doi.org/10.1016/j.socscimed.2019.02.007
Emms, H., Lee, R., Thomas, A., Doerholt, K., Doare, K.L., 2018. Return of vivax malaria in Cyprus. Arch. Dis. Child. https://doi.org/10.1136/archdischild-2018-316459
Goodman, C., Coleman, P., Mills, A., 1999. Cost-effectiveness of malaria control in sub-Saharan Africa. The Lancet 354, 378–385. https://doi.org/10.1016/S0140-6736(99)02141-8
Greenwood, B., Mutabingwa, T., 2002. Malaria in 2002 [WWW Document]. Nature. https://doi.org/10.1038/415670a
Haldar, K., Bhattacharjee, S., Safeukui, I., 2018. Drug resistance in Plasmodium. Nat. Rev. Microbiol. 16, 156–170. https://doi.org/10.1038/nrmicro.2017.161
Hanson, R., 2018. Deciphering Venezuela’s Emigration Wave. NACLA Rep. Am. 50, 356–359. https://doi.org/10.1080/10714839.2018.1550976
Huestis, D.L., Dao, A., Diallo, M., Sanogo, Z.L., Samake, D., Yaro, A.S., Ousman, Y., Linton, Y.-M., Krishna, A., Veru, L., Krajacich, B.J., Faiman, R., Florio, J., Chapman, J.W., Reynolds, D.R., Weetman, D., Mitchell, R., Donnelly, M.J., Talamas, E., Chamorro, L., Strobach, E., Lehmann, T., 2019. Windborne long-distance migration of malaria mosquitoes in the Sahel. Nature 574, 404–408. https://doi.org/10.1038/s41586-019-1622-4
Jaramillo-Ochoa, R., Sippy, R., Farrell, D.F., Cueva-Aponte, C., Beltrán-Ayala, E., Gonzaga, J.L., Ordoñez-León, T., Quintana, F.A., Ryan, S.J., Stewart-Ibarra, A.M., 2019. Effects of Political Instability in Venezuela on Malaria Resurgence at Ecuador–Peru Border, 2018. Emerg. Infect. Dis. 25, 834–836. https://doi.org/10.3201/eid2504.181355
Lo, E., Lam, N., Hemming-Schroeder, E., Nguyen, J., Zhou, G., Lee, M.-C., Yang, Z., Cui, L., Yan, G., 2017. Frequent Spread of Plasmodium vivax Malaria Maintains High Genetic Diversity at the Myanmar-China Border, Without Distance and Landscape Barriers. J. Infect. Dis. 216, 1254–1263. https://doi.org/10.1093/infdis/jix106
Malaria - Symptoms - NHS [WWW Document], 2018. URL https://www.nhs.uk/conditions/malaria/symptoms/ (accessed 12.13.19).
Menard, D., Dondorp, A., 2017. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 7, a025619. https://doi.org/10.1101/cshperspect.a025619
Milner, D.A., 2018. Malaria Pathogenesis. Cold Spring Harb. Perspect. Med. 8, a025569. https://doi.org/10.1101/cshperspect.a025569
Musoke, D., Karani, G., Ndejjo, R., Okui, P., Musoke, M.B., 2016. Experiences of households using integrated malaria prevention in two rural communities in Wakiso district, Uganda: a qualitative study. Malar. J. 15, 313. https://doi.org/10.1186/s12936-016-1369-4
Musset, L., Heugas, C., Naldjinan, R., Blanchet, D., Houze, P., Abboud, P., Volney, B., Walter, G., Lazrek, Y., Epelboin, L., Pelleau, S., Ringwald, P., Legrand, E., Demar, M., Djossou, F., 2019. Emergence of Plasmodium vivax Resistance to Chloroquine in French Guiana. Antimicrob. Agents Chemother. 63. https://doi.org/10.1128/AAC.02116-18
Newton, P.N., Caillet, C., Guerin, P.J., 2016. A link between poor quality antimalarials and malaria drug resistance? Expert Rev. Anti Infect. Ther. 14, 531–533. https://doi.org/10.1080/14787210.2016.1187560
Obaldia, N., Milhous, W.K., Kyle, D.E., 2018. Reversal of Chloroquine Resistance of Plasmodium vivax in Aotus Monkeys. Antimicrob. Agents Chemother. 62. https://doi.org/10.1128/AAC.00582-18
Paniz-Mondolfi, A.E., Tami, A., Grillet, M.E., Márquez, M., Hernández-Villena, J., Escalona-Rodríguez, M.A., Blohm, G.M., Mejías, I., Urbina-Medina, H., Rísquez, A., Castro, J., Carvajal, A., Walter, C., López, M.G., Schwabl, P., Hernández-Castro, L., Miles, M.A., Hotez, P.J., Lednicky, J., Morris, J.G., Crainey, J., Luz, S., Ramírez, J.D., Sordillo, E., Llewellyn, M., Canache, M., Araque, M., Oletta, J., 2019. Resurgence of Vaccine-Preventable Diseases in Venezuela as a Regional Public Health Threat in the Americas. Emerg. Infect. Dis. 25, 625–632. https://doi.org/10.3201/eid2504.181305
Parker, W., 2018. Community health priorities: Lessons for malaria prevention from Balaka district, Malawi. Malawi Med. J. 30, 99–102. https://doi.org/10.4314/mmj.v30i2.9
Rieckmann, K.H., Davis, D.R., Hutton, D.C., 1989. PLASMODIUM VIVAX RESISTANCE TO CHLOROQUINE? The Lancet, Originally published as Volume 2, Issue 8673 334, 1183–1184. https://doi.org/10.1016/S0140-6736(89)91792-3
Rodríguez-Morales, A.J., Suárez, J.A., Risquez, A., Delgado-Noguera, L., Paniz-Mondolfi, A., 2019. The current syndemic in Venezuela: Measles, malaria and more co-infections coupled with a breakdown of social and healthcare infrastructure. Quo vadis? Travel Med. Infect. Dis. 27, 5–8. https://doi.org/10.1016/j.tmaid.2018.10.010
Smith, S., Anker, M., Anker, R., 2017. Living Wage for Lower Volta Region, Ghana [WWW Document]. Glob. Living Wage Coalit. URL https://www.globallivingwage.org/living-wage-benchmarks/ghana/ (accessed 12.13.19).
Snow, R.W., Craig, M., Deichmann, U., Marsh, K., 1999. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull. World Health Organ. 17.
Sriwichai, P., Karl, S., Samung, Y., Kiattibutr, K., Sirichaisinthop, J., Mueller, I., Cui, L., Sattabongkot, J., 2017. Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: cross-border malaria transmission scenario in northwestern Thailand. Malar. J. 16, 258. https://doi.org/10.1186/s12936-017-1900-2
Thu, A.M., Phyo, A.P., Landier, J., Parker, D.M., Nosten, F.H., 2017. Combating multidrug-resistant Plasmodium falciparum malaria. FEBS J. 2569–2578. https://doi.org/10.1111/[email protected]/(ISSN)1742-4658.Malaria
Tuteja, R., 2007. Malaria − an overview. FEBS J. 274, 4670–4679. https://doi.org/10.1111/j.1742-4658.2007.05997.x
Wangdi, K., Gatton, M.L., Kelly, G.C., Clements, A.CA., 2015. Chapter Two - Cross-Border Malaria: A Major Obstacle for Malaria Elimination, in: Rollinson, D., Stothard, J.R. (Eds.), Advances in Parasitology. Academic Press, pp. 79–107. https://doi.org/10.1016/bs.apar.2015.04.002
WHO | WHO commends the Roll Back Malaria Partnership’s contribution to global progress as governing board disbands secretariat [WWW Document], 2015. . WHO. URL https://www.who.int/malaria/news/2015/governing-board-disbands-rbm-secretariat/en/ (accessed 12.4.19).
World malaria report 2019 [WWW Document], 2019. URL https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019 (accessed 12.10.19).
Yaya, S., Uthman, O.A., Amouzou, A., Bishwajit, G., 2018. Mass media exposure and its impact on malaria prevention behaviour among adult women in sub-Saharan Africa: results from malaria indicator surveys. Glob. Health Res. Policy 3, 20. https://doi.org/10.1186/s41256-018-0075-x
Yayon, A., Timberg, R., Friedman, S., Ginsburg, H., 1984. Effects of Chloroquine on the Feeding Mechanism of the Intraerythrocytic Human Malarial Parasite Plasmodium falciparum1. J. Protozool. 31, 367–372. https://doi.org/10.1111/j.1550-7408.1984.tb02981.x
Young, M.D., Moore, D.V., 1961. Chloroquine Resistance in Plasmodium Falciparum. Am. J. Trop. Med. Hyg. 10, 317–320. https://doi.org/10.4269/ajtmh.1961.10.317
Quite a write here, well detailed and fully educative.
Great write up
Wow, really interesting post. I read it all and kearned so many things.
We definitely need more high quality post like this on Steem!
Thank you @suesa.
Thanks for the compliment :)
I wrote this for class and recently received my grade, so I thought why let it go to waste?
@tipu curate
Upvoted 👌 (Mana: 0/10 - need recharge?)
Very good and accurated study. Congratulations.
This post has been voted on by the SteemSTEM curation team
and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider using the steemstem.io app and/or including @steemstem in the list of beneficiaries of this post. This could yield a stronger support from SteemSTEM.