
pixabay.com
Introduction
Monoclonal Antibodies (mAbs) can be used for a variety of things in research and healthcare. From immunoassays to the treatment of cancer and the modulation of a patient’s immune response (Waldmann, 1991), they have a high potential.
The four types of mAbs are murine, chimeric, humanised, and human mAbs, which have an increasing number of regions that are human and are thus less likely to elicit an allergic reaction (National Institute for Cellular Biotechnology, 2016).
When using mAbs, one major problem is their large size of approximately 150 kDa and their relative instability (Kolkman and Law, 2010). These two characteristics limits the administration and use of mAbs considerably. In the previous decades, nanobodies have been developed as an alternative to the classic mAbs because they do not come with the same problems.
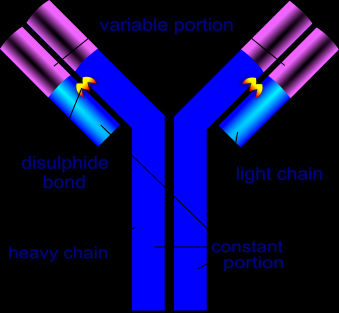
Antibody structure
Credit: wikipedia.org
Attribution-Share Alike 3.0 Unported license.
Nanobodies have been discovered in dromedaries and can also be found in llamas. In contrary to normal antibodies which consist of a light and a heavy chain, nanobodies are only made up of heavy chains, making nanobodies single-domain antibodies. The variable region of these heavy chains is able to bind antigens without the help of a light chain variable region. The lack of a light chain makes nanobodies considerably smaller; their approximate size is only about 15 kDa (Kolkman and Law, 2010).
The smaller size and simpler structure of nanobodies make them not only easier to use and manipulate but also comes with a variety of other advantages that make them a valuable alternative to mAbs. Additionally, it has been demonstrated that nanobodies have a lower potential for immunogenicity, decreasing potential dangers that accompany their usage inside patients (Oliveira et al., 2013).
The following essay will discuss the production, advantages, and use of nanobodies to illustrate their importance in clinical research and the treatment of diseases.
Discussion
Production of nanobodies
While there can be slight variations in how nanobodies are produced, the primary way of production starts with the immunisation of a llama or a dromedary. The animal is injected with the antigen of choice to elicit an immune response, and the blood serum is tested to confirm a sufficiently high titre of the wanted antibodies.
From the immunised animal’s bone marrow, RNA from antibody-producing lymphocytes is isolated and used for the generation of cDNA. These templates are then cloned into a phage vector. After confirmation that the nanobody genes are present in the vector, the phages are then used to infect the cells selected for nanobody expression, for example, Escherichia coli. A colony PCR verifies the successful integration of the nanobody genes in the genome of the nanobody producing cells, which are then put into culture. In a final step, the nanobodies are tested for their antigen-binding efficiency (Fridy et al., 2014; Zare et al., 2014).
The method briefly described above is known as antibody phage display and is not unique to nanobodies. Instead, it is a popular method to generate traditional mAbs, too (Hammers and Stanley, 2014). However, one advantage nanobodies have over mAbs are the expression systems that can be used for their production.
Advantages of nanobodies
Aside from the previously mentioned expression in E. coli, nanobodies can be effectively expressed in Saccharomyces cerevisiae (Frenken et al., 2000) and, even more efficiently, Pichia pastoris (Rahbarizadeh et al., 2006). For expression in yeast cells, the transfection with the nanobody cDNA via phages is replaced with a different vector-specific to yeast.
The efficient production of nanobodies in microbial system sets them apart from mAbs and makes them a cheaper choice, reducing the cost 2 to 3 times compared to expression in mammalian cell lines (Kolkman and Law, 2010).
A few problems with yeast as expression organism remain, although not on a scale that they make this approach unfeasible. Improper folding of the proteins, differences in production efficiency based on cell culture density, and a significant accumulation of nanobodies inside the cell instead of being secreted are the main problems that need to be solved (Frenken et al., 2000).
The single-domain nature of nanobodies allows for easy genetic manipulation and makes the production of multivalent forms less complicated, as there can be no accidental mispairing of the light and the heavy chain. Beyond that, nanobodies exhibit higher physiochemical stability and higher solubility than mAbs, and their small size enables them to penetrate tissue much faster and recognize even hidden antigenic sites (Harmsen and De Haard, 2007).
Easy and fast tissue penetration is especially crucial in the treatment of cancer with solid tumours (Xie et al., 2019). Due to their size, mAbs tend to not penetrate tumour tissue efficiently, while the smaller nanobodies can be expected to be 10 times faster (Kolkman and Law, 2010)
How nanobodies are used
Cancer therapy is one of the main applications for nanobodies and will thus be the main topic of this section. The World Health Organization reported cancer to be the second leading cause of death globally in 2018, with 1 in 6 deaths being due to cancer (WHO, 2018).
In one mouse study, nanobodies were able to selectively target tumours that produced hepatocyte growth factor which resulted in the inhibition of tumour growth and eventually fully cured the cancer (Vosjan et al., 2012).
A less specific antigen against which nanobodies have been demonstrated to be effective is CD47, a protein that is involved in the escape of tumour cells from the immune system. The CD47/SIRPα signal pathway can be blocked by an anti-CD47 nanobody, leading to phagocytosis of tumour cells by macrophages. In vivo, this approach worked to combat both ovarian cancer and lymphoma (Ma et al., 2020).
There are many more examples for the potential anti-cancer application of nanobodies. An in E. coli expressed nanobody against human papillomavirus 16 was able to inhibit the proliferation of cells infected with the virus (Li et al., 2019), and a nanobody against metastatic colorectal cancer expressing EGFR (epidermal growth factor receptor) was even able to overcome resistance against previously used antibodies (Tintelnot et al., 2019).
One interesting approach to nanobody immunotherapy is the use of lactic acid bacteria that are used as a vehicle to deliver nanobodies in situ. In one study, the lactic acid bacteria successfully delivered the nanobodies to the gastrointestinal tract (GIT) of human test subjects, giving the opportunity to address GIT inflammatory diseases and cancers (del Rio et al., 2019). Lactobacillus paracesei was already used to deliver nanobodies designed to fight the endotoxins produced by Clostridium difficile, a bacterium that can cause diarrhoea (Andersen et al., 2016).
But nanobodies do not necessarily need to neutralise a toxin or block a pathway to be effective against cancer. Some approaches fuse nanobodies with other molecules like, for example, branched gold nanoparticles. Nanobodies bioconjugated with these nanoparticles can bind to a tumour cell, and laser irradiation of the gold produces an amount of heat sufficient to destroy the targeted cells (Broek et al., 2011).
Beyond therapeutic use, nanobodies can also be used for non-invasive imaging of tumours by binding to extracellular matrix biomarkers typical for cancers (Jailkhani et al., 2019), and non-invasive detection of cells without affecting their viability or function (Demine et al., 2020). The ability to image tumours and detect cells without surgical intervention has the potential to reduce the stress put on a patient whose body is already struggling with cancer.
Conclusion
Nanobodies are versatile molecules with many advantages over traditional mAbs and a variety of possible applications.
Cancers are one of the top global leading causes of death. Developing cheap, non-invasive, and non-damaging cures for the many different types is a major goal that can possibly be reached with nanobodies thanks to their unique features.
Especially the small size and relatively easy and cheap production is what makes nanobodies so special and such a valuable tool to not only combat a multitude of cancers, but also to deliver other molecules and help with tumour imaging. It is to be seen what other applications may eventually be developed.
A few existing problems still need to be tackled, like the previously discussed downsides of using yeast as an expression system. One downside nanobodies share with mAbs is that they require multiple steps before they can be produced in culture, usually involving an animal that needs to be immunised first. The process can be long and expensive and may not even be successful in the end.
Still, the sheer number of studies and cancers that could be addressed by using nanobodies warrant further research into them. Nanobodies as a tool for research and therapy have a lot of potential and a promising future ahead.
Bibliography
Andersen, K.K., Strokappe, N.M., Hultberg, A., Truusalu, K., Smidt, I., Mikelsaar, R.-H., Mikelsaar, M., Verrips, T., Hammarström, L., Marcotte, H., 2016. Neutralization of Clostridium difficile Toxin B Mediated by Engineered Lactobacilli That Produce Single-Domain Antibodies. Infect. Immun. 84, 395–406.
Broek, B.V. de, Devoogdt, N., D’Hollander, A., Gijs, H.-L., Jans, K., Lagae, L., Muyldermans, S., Maes, G., Borghs, G., 2011. Specific Cell Targeting with Nanobody Conjugated Branched Gold Nanoparticles for Photothermal Therapy
del Rio, B., Redruello, B., Fernandez, M., Martin, M.C., Ladero, V., Alvarez, M.A., 2019. Lactic Acid Bacteria as a Live Delivery System for the in situ Production of Nanobodies in the Human Gastrointestinal Tract. Front. Microbiol. 9.
Demine, S., Garcia Ribeiro, R., Thevenet, J., Marselli, L., Marchetti, P., Pattou, F., Kerr-Conte, J., Devoogdt, N., Eizirik, D.L., 2020. A nanobody-based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice. Diabetologia 63, 825–836.
Frenken, L.G.J., van der Linden, R.H.J., Hermans, P.W.J.J., Bos, J.W., Ruuls, R.C., de Geus, B., Verrips, C.T., 2000. Isolation of antigen specific Llama VHH antibody fragments and their high level secretion by Saccharomyces cerevisiae. J. Biotechnol. 78, 11–21.
Fridy, P.C., Li, Y., Keegan, S., Thompson, M.K., Nudelman, I., Scheid, J.F., Oeffinger, M., Nussenzweig, M.C., Fenyö, D., Chait, B.T., Rout, M.P., 2014. A robust pipeline for rapid production of versatile nanobody repertoires. Nat. Methods 11, 1253–1260.
Hammers, C.M., Stanley, J.R., 2014. Antibody Phage Display: Technique and Applications. J. Invest. Dermatol. 134, e17.
Harmsen, M.M., De Haard, H.J., 2007. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 77, 13–22.
Jailkhani, N., Ingram, J.R., Rashidian, M., Rickelt, S., Tian, C., Mak, H., Jiang, Z., Ploegh, H.L., Hynes, R.O., 2019. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. 116, 14181–14190.
Kolkman, J.A., Law, D.A., 2010. Nanobodies – from llamas to therapeutic proteins. Drug Discov. Today Technol. 7, e139–e146.
Li, S., Zhang, W., Jiang, K., Shan, H., Shi, M., Chen, B., Hua, Z., 2019. Nanobody against the E7 oncoprotein of human papillomavirus 16. Mol. Immunol. 109, 12–19.
Ma, L., Zhu, M., Gai, J., Li, G., Chang, Q., Qiao, P., Cao, L., Chen, W., Zhang, S., Wan, Y., 2020. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J. Nanobiotechnology 18, 12.
National Institute for Cellular Biotechnology, 2016. What is a Monoclonal Antibody? [WWW Document]. Natl. Inst. Cell. Biotechnol. URL https://nicb.ie/biotechnology/what-is-a-monoclonal-antibody/ (accessed 3.22.20).
Oliveira, S., Heukers, R., Sornkom, J., Kok, R.J., van Bergen en Henegouwen, P.M.P., 2013. Targeting tumors with nanobodies for cancer imaging and therapy. J. Controlled Release 172, 607–617.
Rahbarizadeh, F., Rasaee, M.J., Forouzandeh, M., Allameh, A.-A., 2006. Over expression of anti-MUC1 single-domain antibody fragments in the yeast Pichia pastoris. Mol. Immunol. 43, 426–435.
Tintelnot, J., Baum, N., Schultheiß, C., Braig, F., Trentmann, M., Finter, J., Fumey, W., Bannas, P., Fehse, B., Riecken, K., Schuetze, K., Bokemeyer, C., Rösner, T., Valerius, T., Peipp, M., Koch-Nolte, F., Binder, M., 2019. Nanobody Targeting of Epidermal Growth Factor Receptor (EGFR) Ectodomain Variants Overcomes Resistance to Therapeutic EGFR Antibodies. Mol. Cancer Ther. 18, 823–833.
Vosjan, M.J.W.D., Vercammen, J., Kolkman, J.A., Walsum, M.S., Revets, H., Dongen, G.A.M.S. van, 2012. Nanobodies Targeting the Hepatocyte Growth Factor: Potential New Drugs for Molecular Cancer Therapy. Mol. Cancer Ther. 11, 1017–1025.
Waldmann, T., 1991. Monoclonal antibodies in diagnosis and therapy. Science 252, 1657–1662.
WHO, 2018. Cancer [WWW Document]. URL https://www.who.int/news-room/fact-sheets/detail/cancer (accessed 3.22.20).
Xie, Y.J., Dougan, M., Jailkhani, N., Ingram, J., Fang, T., Kummer, L., Momin, N., Pishesha, N., Rickelt, S., Hynes, R.O., Ploegh, H., 2019. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. 116, 7624–7631.
Zare, H., Rajabibazl, M., Rasooli, I., Ebrahimizadeh, W., Bakherad, H., Ardakani, L.S., Gargari, S.L.M., 2014. Production of Nanobodies against Prostate-Specific Membrane Antigen (PSMA) Recognizing LnCaP Cells. Int. J. Biol. Markers 29, 169–179.
Thanks for this. It seems clear (from your post) that those nanobodies (I have never heard that term before today) can be used in mouse, after being extracted from llamas or dromedaries. However, I can imagine we are far from being able to use them for humans, keeping in mind a path to cure cancer. Do you have some information on how far we are on this journey?
Last year one has been approved by the FDA against a bloodclotting disorder and there's one being worked on against cancer in the EU, supposed to be ready summer 2021 (although with the ongoing pandemic, that may be delayed).
Thanks for the information (and the links => some readings for the next few days).
This post has been voted on by the STEMsocial curation team and voting trail. It is eligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
For additional information please join us on the STEMsocial discord and to get to know the rest of the community!
Please consider using the stem.openhive.network app and including @steemstem as a beneficiary of this post. This could yield a stronger support.