Hello friends of steemit and steemstem, today I want to talk a little about the semiconductor materials that are fundamental in the construction of electronic devices that we use every day.
Source
A semiconductor is a crystalline substance that at the temperature of absolute zero has a structure of energy bands in which a band of fully filled electronic states is separated from one that is completely empty by means of a narrow region of forbidden energies.
At absolute zero, the semiconductor is a perfect insulator since it does not have partially full bands. However, at higher temperatures, some electrons in the valence band can acquire enough random thermal energy to be excited through the forbidden band in order to become driving electrons in the previously empty driving band.
The empty states remaining in the lower or valence band can also contribute to conductivity, behaving as positively charged gaps. It is evident that the number of conduction electrons and the number of holes must rise when the temperature increases and, therefore, the electrical conductivity also increases with increasing temperature.
The semiconductor is a material that has a level of conductivity at some point between the ends of an insulator and a conductor, that is, it is a material that under certain conditions turns out to be an insulator, but under other conditions it turns out to be a conductor, hence its name .
Semiconductors are mainly classified into two categories: Intrinsic and Extrinsic.
An intrinsic semiconductor is known as the one where holes and electrons are created exclusively by thermal excitation through the forbidden band of energy. Holes and electrons created in this way are often called charge carriers and the conductivity caused by these carriers is called intrinsic conductivity.
In an intrinsic semiconductor, the concentrations of electrons and holes must always be the same, since the thermal excitation of an electron inevitably causes only a hole.
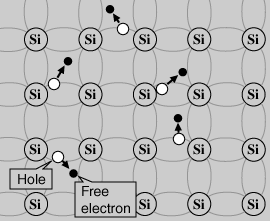
intrinsic semiconductor
The characteristics of the semiconductor materials can be significantly altered by the addition of certain impurity atoms such as arsenic, antimony or other elements belonging to group V of the periodic table, to a relatively pure (intrinsic) semiconductor material such as silicon or germanium crystals. , these impurities, even if only 1 part in 10 million has been added, can sufficiently alter the structure of the band and completely change the electrical properties of the material. On the other hand, a semiconductor material that has been doped is called an extrinsic material.
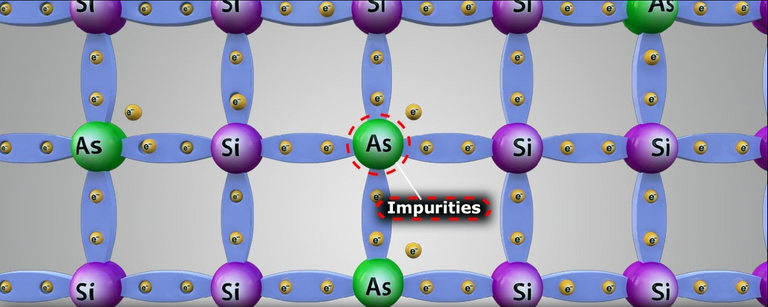
extrinsic semiconductor
n-TYPE AND p-TYPE MATERIALS
Both type n material and type p are formed by the addition of a predetermined number of impurity atoms to the pure semiconductor.
The n-type material is created through the introduction of impurity elements that donate additional electrons and therefore they are called donor atoms since each of them donates an additional free electron to the crystal.
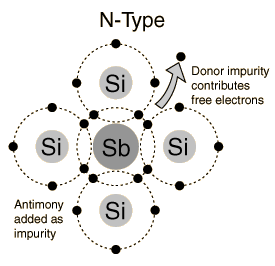
extrinsic material type n
The p-type material is formed by doping a pure crystal, for example of germanium or silicon, with impurity atoms possessing three group III valence electrons from the periodic table.
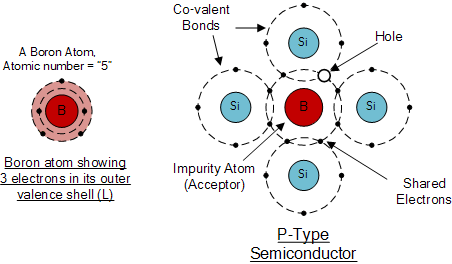
extrinsic material type p
Type n and p materials represent the basic building blocks of semiconductor devices.
REFERENCES:
Kittel, C. Introduction to solid state physics, John Willey & sons, INC. New York. 1954.
Hall, H. E. Física del estado sólido, editorial Limusa. México D.C. 1978.
Mckelvey, J. Física del estado sólido y de semiconductores. Editorial Limusa. México D.C. 1976
S.M. Sze. Physics of semiconductor devices, Weley-Interscience, second edition. 1981.