Overview
The scope of this post is to provide a fundamental understanding of what heat is and how it is transferred. The post describes heat as energy, and defines temperature as a measure of heat energy.
What is Heat?
As mentioned above heat is energy, and is transferred from one state to another such that there is equilibrium. In other words when two materials come into contact that have different levels of heat energy, the hotter material transfers energy to the cooler one. Heat is always transferred in this manner. For example heat exchanges work to cool systems by moving heat away from the system. Heat sinks work in a similar fashion by moving heat away from components like microprocessors and transistors.
Matter is made up of atoms and molecules that are in motion. When heat is added the atoms vibrate faster in the form of kinetic energy. As atoms vibrate faster the space between atoms increase creating potential energy.
Solids, liquids, and gasses all expand when heat is added. When heat leaves a substance it is known as cooling, and the molecules vibrate slower and matter contracts.
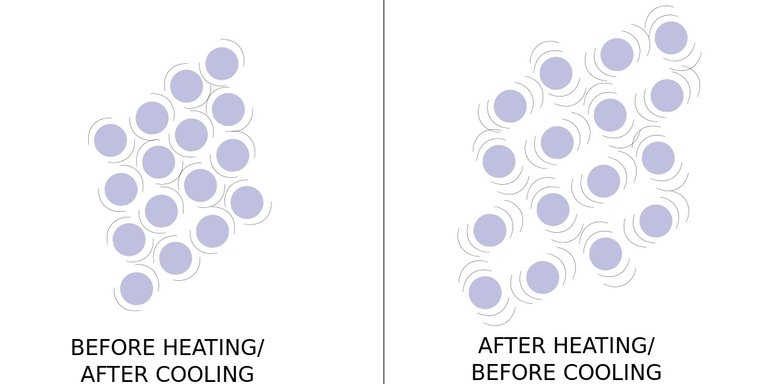
Image Source: By Kayau (Own work) [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
When exposed to heat materials can change state, causing a chemical reaction or a phase transition. A phase transition occurs when enough heat is applied or removed, such that a materiel changes from one form to another. For example heat energy causes a solid to become a liquid, and a liquid to becomes a gas. The process is also reversible, as a gas cools it condensates into a liquid, and when a liquid cools it turns into a solid.
Heat can be a factor in chemical reactions like combustion. For example with enough heat materials combine with oxygen and burn, and gases explode.
How is Heat Transferred
There are three general classifications of how heat is transferred including: Convection, Conduction, and Radiation.
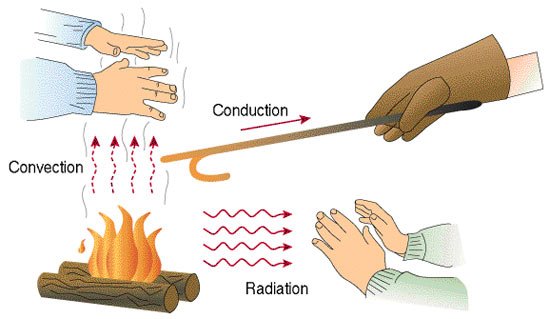
By Kmecfiunit (Own work) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
Conduction occurs when the transfer of heat (internal energy) is caused by microscopic collisions of particles and movement of electrons within a body. The picture above shows a metal rod with the tip applied to a heat source, conduction occurs in the rod as heat is transferred from the hot to the cooler end. The picture below shows an example where a burner is in direct contact with the kettle, so heat energy is transferred from the burner.
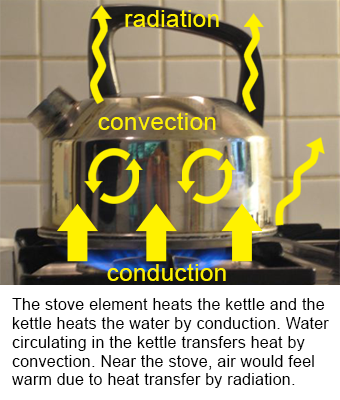
By P.wormer [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Conduction takes place in all phases of matter, including solids, liquids, gases and waves. However solids are better conductors than liquids, and liquids are better conductor than gases. Some metals have better conductive properties than others. For example copper conducts heat better than aluminum, and aluminum better than bronze.
Convection happens in fluids but does not happen in solids. Liquid or gas in hot areas are less dense than the liquid or gas in cold areas, causing them to rise. The denser cold fluids are displaced and fall into the warm areas. This creates circulating convection currents that transfer heat.
One example of convection is the bubbles of water in boiling water. The hotter parts of the water are rising to the cooler area of water at the top of the pan. The cooler area of water is pushed down where it is then heated creating a cycle of convection current.
Convection occurs in the atmosphere, as hot air rises and cool air falls to take its place. Heat energy is transferred by the circulation of the air.
Convection does not occur in solids because the particles within are too tightly packed to facilitate the process. Convection requires actual movement between the particles within a substance in order to transfer heat which is only possible in a fluid state of matter such as liquid or gas.
Radiation is a method of heat transfer that does not require contact between the heat source and the object being heated. In the pictures above radiation is shown as the air surrounding the heat source is warmed by the transmission of heat energy through space.
The sun radiates heat energy as we feel the heat and are not in direct contact. Heat is transmitted though space by thermal radiation. Thermal radiation known as infrared radiation (IR) is a type of electromagnetic radiation or light. No mass is exchanged and no medium is required.
What is Temperature?
While relating to heat energy, in that more heat relates to a higher temperature, temperature is not the same as heat. Heat has the ability to do work, where temperature is a measure of the degree of heat. Temperature determines the direction in which heat will flow.
Heat is symbolized by the letter Q and is a type of energy that is measured in Joules (J). Heat is energy that is transferred from one body to another as the result of a difference in temperature.
Temperature symbolized by the letter T is a measure of hotness or coldness. It relates to the average motions of a single particle, per degree of freedom. Temperature is commonly measured in degrees of Kelvin(K), Celsius ©, or Fahrenheit (F).
Further Investigation
This post is inspired by a desire to publish articles on measuring temperature. I do a lot of work with both contact temperature measurement using thermocouples, and non contact measurements with pyrometers.
It is interesting to note that just as molecules spin faster with heat, that they move slower with an absence of heat. When you put food in the refrigerator, you remove heat and slow down the chemical and biological process relating to decay and bacteria growth.
If you continue to remove all the heat, until there is no heat energy, you reach a state of absolute zero. This is a theoretical place, but has been approached in labs. Some interesting things happen as you approach absolute zero, including materials loosing resistance to electrical current and creating the property of a super conductor.
I will leave it here for now. What are your thoughts? Is there anything about heat energy, temperature, or absolute zero you can add to this post? Is there any area you are interested in that I can pick up on in my next effort?
Thanks for reading. Let me know your thoughts! --3D
Sources
All sources reflect state of website in Feburary 2018
I. https://www.mansfieldct.org/Schools/MMS/staff/hand/atomsheat.htm
II. http://coolcosmos.ipac.caltech.edu/cosmic_classroom/light_lessons/thermal/
III. https://en.wikipedia.org/wiki/Heat_transfer
IV. http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/energy/heatrev1.shtml
V. https://www.diffen.com/difference/Heat_vs_Temperature
VI. https://en.wikipedia.org/wiki/Temperature
VII. http://www.explainthatstuff.com/heat.html
Being A SteemStem Member
Very nice post, I enjoyed reading it!
Even though I knew all these stuff it kept me interested and very informational.
I like how you explained conduction, convection and radiation with fire and kettle - my professor explained it with spoon in the boiling water, you reminded me of him. :)
Maybe there could be a bit more about phase transition and latent heat, but that could also be one of possible post in the future.
73, Velibor :)
Thank you for the kind words! I agree that much more could be said on phase transition, as that topic alone would make a great post. I could fill some of the gaps in my knowledge. It is all very fascinating.
--73, Joe
Congratulations @three-d! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP