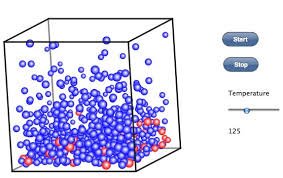
Hello steemit today I will be sharing my knowledge in other words what I think about the molecular explanation of temperature
The molecules that makes up a solid vibrate about a fixed position. When heat is applied to the solid, the amplitude of vibration or these molecules become larger, and invariably their kinetic energy (K.E) increases. From kinetic theory, the total translational kinetic energy of the molecules is directly proportional to the absolute temperature, hence increase in kinetic energy implies increase in the temperature of the molecules. This means that increase in temperature means increase in kinetic energy and verse versa. Both depend on the quantity of heat supplied to a body being heated, a body of smaller mass will get heated up easily than that with a larger mass if same quantity of heat is supplied. This is true because the number of molecules contained in the former is less than that contained in the latter.
Please guys if you don't understand don't hesitate to ask at the comment section
Written by
@thunderstruck1