Hello friends of Steemit!
This time I'm talking about one of the most controversial moments of physics and intrigue, at least for me, a solution proposed by one of the greatest physics scientists, known by many as the father of quantum mechanics, Max Planck.
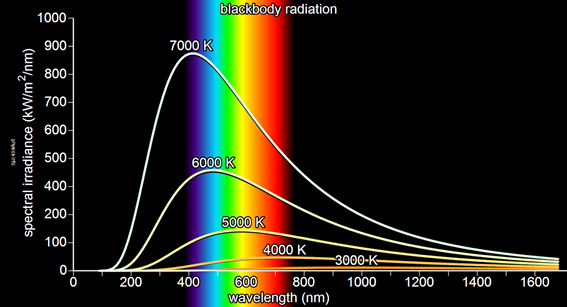

Thermal radiation was the beginning of a series of studies that by the time of 1900 had the attention of many scientists.
The bodies whose surfaces absorb all the thermal radiation that impinges on the os are called black bodies. Physicist Gustav Kirchhoff is the first to introduce the concept of the black body. The radiation properties of the so-called black body were always of interest to physicists who sought an explanation of their spectral behavior. Based on an imaginary model, Gustav Kirchhoff manages to demonstrate that the intensity of radiation depends on the temperature.
Other blackbody models were taken as reference for spectral analysis. A practical model was one that consisted of a cavity with a small hole. The radiation coming from the outside penetrates through the small hole, reflecting in all the senses and remaining confined in the cavity. Since the area of the hole is very small, all the radiation is absorbed, converting the body into a perfect absorbent.
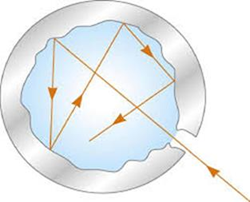
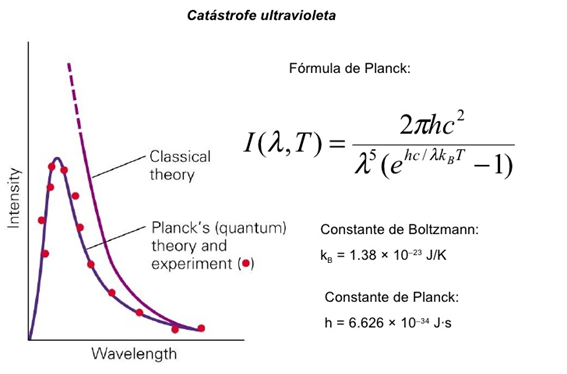
Many scientists tried to explain the distribution of blackbody radiation based on classical principles. But all of them failed because the theoretical models did not fit the experimental data.
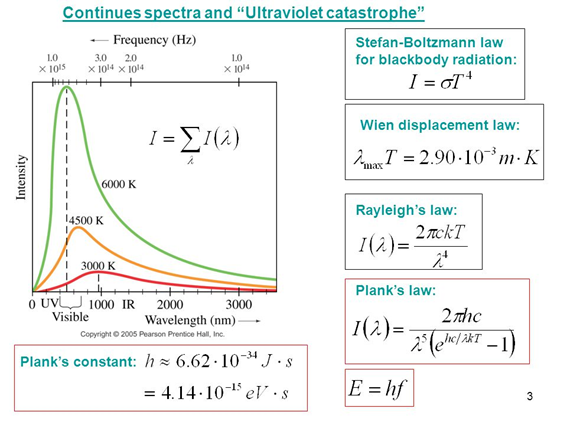
Rayleigh and Jeans attempted to calculate the average total energy of the frequency-dependent standing waves. According to classical physics, energy could have any value between zero and infinite. Serious conflicts arose with classical laws, such as the equipartition theorem and the existing kinetic theory. The discrepancy between the values obtained by his model and the experimental results were evident.
Only an approximation of the classical model with the results at low frequencies is observed, the behavior so sharply removed from the theoretical model and the experimental results for large frequency values is what we know as the ultraviolet catastrophe.

A failure of classical theories was evident.
Many works aimed at explaining such radiation phenomena. Scientists such as Stefan, Boltzmann, Wilhelm Wien also postulated their theories, but there were certain discrepancies in the infrared region of the spectrum.
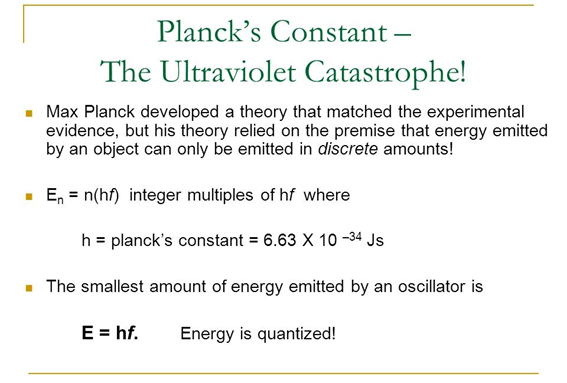
It is Max Planck who proposes a revolutionary idea that solves the problem. An idea with which even he was not so happy because of the implications it had. Planck himself considered his postulate as a desperate act. This is the moment in which quantum physics is born.
The great contribution of Planck was to realize that the experimental and theoretical results only coincided if energy was treated as a discrete variable instead of a continuous variable as defined by the classical model.
Using numerical analysis finds a simple proportionality between the energy and the frequency of radiation of the form:
Where:
"h" is the constant of proportionality
Planck calculates the value of said constant in such a way that the experimental results fit his model.
In this way the concept of quantization of energy is introduced.
We could ask where was "h" until that moment?
The reason is that the value of "h" is so small that in the classic models its value and effect is negligible. Quantum models can be reduced to classic models by simply making "h" store to zero.

I hope that the review of the so-called Ultraviolet Catastrophe and the proposed solutions will serve to nourish your knowledge on the subject. Happy day.
May I suggest that you added some references to your post? References help a lot in further studying of the topic and add up to a post's credibility ;)
Thanks for the suggestion, I will keep it in mind in my future publications. It is a fascinating and widely documented subject that I wanted to share. Thanks for your support and comment. good night