Author:
Christopher M. Brainard, Esq. [[email protected]]
Title:
USE OF RNA ENIGNEERED MESENCHYMAL STEM CELLS IN THE TREATMENT OF HEART DISEASE (2017)
Abstract:
Heart disease remains the leading cause of death worldwide and though treatable by surgeries that forestall the inevitable, it remains a formidably progressive disease. Various stem cell therapies including the use of mesenchymal stem cells have been applied and have been observed to provide benefit though it has been recognized early on that few of the stem cells actually survive long term or differentiate. Early observed benefits and regenerative effects from stem cell applications were determined to have occurred through signaling by the stem cells causing endogenous proliferation and differentiation of mostly host stem cells. New research indicates that engineering stem cells through mRNA-133a or lncRNA-Bvht transfection can allow the applied stem cells to survive longer thereby prolonging their ability to signal endogenous repair processes or to differentiate into cardiogenic cell types capable of replenishing and replacing existing otherwise damaged host cells, respectively. Individually, mRNA-133a has been shown in preventing hypertrophy, preventing fibrosis, and facilitating cardiac remodeling. Individually, lncRNA-Bvht is reported to facilitate stem cell pluripotency and modulation of cardiac differentiation during both development and repair in mouse cardiac injury. This opens up the question as to whether the use of both mRNA-133a and lncRNA-Bvht can be used together to provide even greater benefits than either individually – this paper suggests this hypothesis should be tested. It is logical to conclude based on the data that in the future stem cells will be physically as well as environmentally conditioned in conjunction with various coordinated RNA engineering techniques to achieve a long lasting and more permanent solution to heart disease.
_
USE OF RNA ENIGNEERED MESENCHYMAL STEM CELLS IN THE TREATMENT OF HEART DISEASE IN RODENTS
I. INTRODUCTION
a. LEADING CAUSE OF DEATH OF HUMANS CONTINUES TO BE HEART DISEASE
The leading cause of death worldwide remains the dark specter of heart disease (Fang, et. al., 2016). In fact, heart disease accounts for 23.4 % of all deaths in the United States (Nichols, 2017). The characteristics of heart disease, whether myocardial infarction, ischemia, congestive failure, all involve a progressive process whereby the myocardium fails to fully regenerate, establish sufficient perfusion, or otherwise heal, leading to ever greater reductions in efficiency and cardiac output (Premer, et. al., 2016). Thus, more effective approaches for heart disease remain a top priority and deserve our attention since tremendous strides can be achieved in preserving and extending vast amounts of human life via more effective treatments for this disease.
b. PREVAILING TREATMENT FOR HEART DISEASE REMAINS SURGICAL
The prevailing current treatments for advanced heart disease continues to be surgically oriented and include stents, angioplasty, pace-makers, oblation, and coronary artery bypass grafting as the most common heart surgery. Despite the tremendous advances in surgical treatments and the coordinate decline in cardio vascular mortality, this surgical approach only provides at best a temporary delay in a progressive disease process (Karantalis & Hare, 2015). Indicative of this fact that at most we are buying time through surgery was a study demonstrating average life expectancy after heart transplantation was 9.16 years post operation (Politi, et. al. 2004). This represents a significant amount of time gained, but it remains less than a solution.
For obvious reasons, invasive surgical methods carry relatively mixed results due to inevitable complications related to anesthesia, trauma, long recovery times, scarring, and limited long term survival rates. Surgical solutions also tend to be applied during late stages as an attempt to treat a problem rather than as a preventative measure. In recent years and in response to the need for better approaches, less invasive cell based therapies have emerged as a potentially superior means for addressing the problem.
c. NAÏVE MESENCHYMAL STEM CELLS: THEIR USEFUL APPLICATION AND THEIR LIMITATIONS ABSENT ENGINEERING
i. MSC DEMONSTRATE USEFUL APPLICATION
Due to the fact that mesenchymal stem cells (MSCs) have the ability to differentiate into derived tissues, have immunomodulatory effects, are available from various sources, and can replenish endogenous stem cell niches, they have been identified as potentially the most promising and certainly one of the most investigated and clinically tested type of stem cell (Karantalis & Hare, 2015). As an MSC the cell must express surface molecules CD73, CD90, and CD105, in the absence of surface molecules CD34, CD45, HLA-DR, CD14 or CD11b, CD79a, or CD19 (Karantalis & Hare, 2015). CD73 is believed to facilitate binding and engraftment, facilitate in MSC migration, and adaptive MSC immunomodulation (Airas, et. al. 1997). CD73 as well as CD90 is strongly positively expressed on MSCs as is CD105 prior to differentiation, at which time these surface markers decrease (Boxal & Jones, 2012). Similarly, we know that the pluripotency disappears when cells begin to differentiate and express markers CD34, CD45, HLA-DR, CD14 or CD11b, CD79a, or CD19 (Airas, et. al. 1997).
There are a variety of sources for MSCs with derivation from adipose, bone marrow, and umbilical cord as the most common and pervasive in clinical trials. Adipose and bone marrow derivation of MSCs can result in autologous MSCs that are genetically identical to the host and as such provide a good theoretical basis for replenishing dysfunctional or malfunctional cell types, while umbilical cord derived MSCs are allogeneic. Allogeneic cells are from a donor and are not genetically identical to the host recipient and as such would imply the potential for host graft disease or other rejection issues could arise. Nevertheless, allogeneic MSCs even across species (human to mice) have been used successfully as a favorable prophylactic for host graft disease demonstrating that the immune system modulating effects provided by MSCs are extremely powerful and lead to the conclusion that prior to differentiation, allogeneic MSCs are useful without out risk of rejection (Wang, et. al. 2016). The methods for what the exact signals are that cause suppression at the situs of immune response and rejection is based on cytokines, paracrine, and other messengers and complex interplay as they are released by the allogeneic MSCs, but is not yet fully understood and deserve further study.
In practice and observation, umbilical derived MSCs have been very useful despite the fact they are allogeneic due to the immunomodulatory properties that appear to be endemic to MSC types. MSCs have been observed to suppress localized white blood cells, inhibit T-cell proliferation, and suppress inflammation locally allowing the MSCs to provide benefit without rejection (Karantalis & Hare, 2015). Again, these are observed events and the exact mechanism is still not fully understood with regards to the complex signals that prevent host rejection of the allogeneic MSCs. (Observed in Wang, et. al. 2016). What we know is in essence, the host immune system is signaled by the MSCs into not rejecting the MSCs, thereby allowing these allogeneic MSCs to engage in a command and control function whereby they signal toward healing and regeneration and whereby they engraft for some time in an area in need of repair and then signal local host cells inclusive of host stem cells to proliferate and differentiate. (See Singh, et. al., 2016; see also Sarkissian, et. al., 2017)).
The success in application of allogeneic MSCs has proved to be a more effective treatment option as compared to autologous MSCs in restoring endothelial function heart failure by stimulating endothelial progenitor cells in humans (Premer, et. al., 2015). Autologous MSCs are normally derived from the host bone marrow, adipose, or other tissues and have the benefit of being genetically identical to the host. Nevertheless, this allogeneic advantage may be attributed to the fact that the autologous MSCs used in treating patients with advanced heart disease are already relatively mature as compared to umbilical cord derived MSCs which by definition are a close approximation to embryonic stem cells (ESCs) and likely have a higher degree of effective potency via the ability to differentiate into more tissue types and have higher and more effective intercellular communications and biomolecular commands since both ESCs and MSCs secrete a large variety of cytokines, growth factors, and extracellular components that act in an autocrine and/or paracrine manner; cytokines signal and modulate the host immune response while chemokines are involved in chemotaxis, and growth factors stimulate cell growth, proliferation, and differentiation (Sarkissian, et. al., 2017). It is clear that the complex signaling involved with cytokines which modulate how immune response along with the various growth factors that stimulate growth, proliferation, and differentiation are some of the key signaling that the MSCs are providing (Sarkissian, et. al., 2017).
_
II. DATA
a. MSC LIMITATIONS ABSENT ENGINEERING SUFFER POOR SURVIVAL, PROLIFERATION, ENGRAFTMENT, AND DIFFERENTIATION
Despite excitement in recent years surrounding regenerative cellular therapies, success has been limited by the realization that normal reparative processes of the heart are insufficient to restore damaged heart tissue to normal functional capacity and as evidenced in Figure 1 and Figure 2 herein, cellular cardiomyoplasty is hampered by poor survival, proliferation, engraftment, and differentiation of the donated MSC population (Mohsin, et.al., 2011). In Figure 1, we see that as little as 5% of the untreated donated cells survive. In Figure 2, as few as only 10% of the donated cells actually achieve differentiation. While it was originally thought that the donated MSCs were differentiating in substantial quantities, in fact a very few of the donated MSCs in early studies failed to engraft or differentiate and the early observed benefits came as a result of almost entirely by the MSCs secreting a large variety of signals as cytokines, growth factors, and extracellular components that act in an autocrine and/or paracrine manner; cytokines signal and modulate the host immune response while chemokines are involved in chemotaxis, and growth factors stimulate cell growth, proliferation, and differentiation (Sarkissian, et. al., 2017; see also Karantalis & Hare, 2015).
These observations indicated limits to the effectiveness of MSCs since they only provided benefit for as long as they survived and signaled endogenous stem cells to proliferate and differentiate. Host stem cells are a finite resource and like all cells will become old and/or senescent in accord with natural processes including telomere length shortening and as such it is logical to conclude that eventually, donated MSCs that merely command and control will lose effectiveness. Without the ability of the donated MSCs to survive, engraft, differentiate, and replace damaged tissue, the current applications suggest that they too, like surgical interventions which currently pervade, can merely buy time in what is still progressive a disease process.
b. NO STUDY HAS BEEN DONE SHOWING WHETHER mRNA-133a and lncRNA-Bvht CAN BE USED IN CONJUNCTION TO INCREASE SURVIVABILITY AND DIFFERENTIATION IN RODENTS
i. SURVIVABILITY CAN BE INCREASED WITH mRNA-133a
The hypothesis that mRNA-133a can increase the survivability of stem cells has gained support in experiments on rats (Dakhlallah, 2015). mRNA-133a is normally abundant in cardiac tissue, but is down regulated in patients that have suffered myocardial infarction. Consequently, rats were induced to myocardial infarction and then treated with mRNA-133a transfected MSCs versus MSCs or a mRNA-133a inhibitor, and there was a significant in vivo 500% increase in survivability which in turn lead to increases in cell engraftment, cardiac function, and reduced fibrosis with the mRNA-133a mimic transfected MSCs (Dakhlallah, 2015). Though the precise mechanism(s) is a subject of further study, treatment with mRNA-133a has shown in patients with myocardial infarction prevented cardiac hypertrophy, reduced fibrosis, and cardiac remodeling (Dakhlallah, 2015). Notably, application of an antagomir (i.e., an agent that blocked resident activity of mRNA-133a) which inhibited mRNA-133a demonstrated reduced cell viability when compared to untreated MSCs in rodents. See Figure 1.
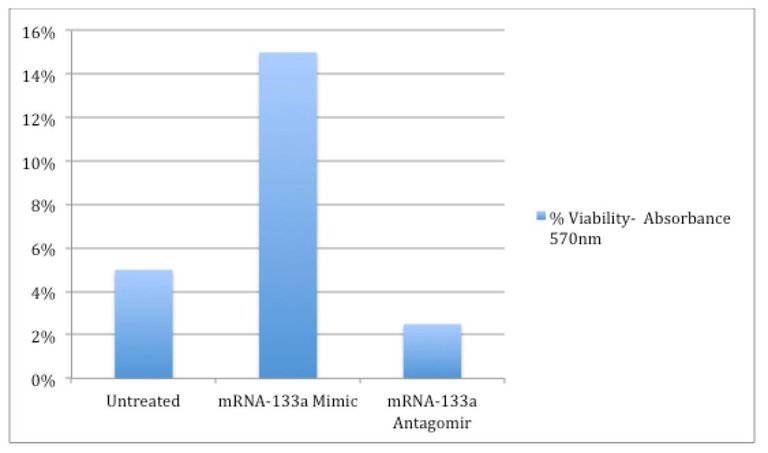
Figure 1: Increased Viability of MSCs In Vivo With mRNA-133a Transfection
ii. DIFFERENTIATION IS INCREASED BY RNA TRASFECTION OF LONG NONCODING RNA BRAVEHEART (lncRNA-Bvht)
Long Noncoding RNA Braveheart (“lncRNA-Bvht) has been identified and utilized successfully in the mouse model as a regulator of cardiac tissue specification and differentiation (Hou, et. al., 2017). Long noncoding RNA (lncRNA) orchestrate specific genes and act in a regulatory fashion that affect many processes including cell development, stem cell pluripotency, and disease etiology (Hou, et. al., 2017). lncRNA-Bvht normally is associated during mouse embryonic stages in facilitating cardiac specification and differentiation (Hou, et. al., 2017). As such, though the exact mechanism is not yet clear in the fully mature heart, the observed effectiveness of lncRNA presumes the same signals provided in the embryonically developing heart also provide another different avenue to mRNA in activating cellular process that can potentially augment and increase viability and differentiation in the mature model (Hou, et. al., 2017).
Exemplary, in three-week-old mice, femoral bone marrow was extracted and it was shown that in vitro differentiation into cardiogenic tissue was increased by transfection with lncRNA-Bvht (Hou, et. al., 2017). The data demonstrated cadiogenic differentiation based on the expression of the cardiac markers including “cTnT.” After 14 days of stimuli in culture of lncRNA-Bvht, MSCs displayed mature cardiogenic phenotype as evidenced by increased expression of marker cTnT. cTnT is a marker expressed by cardiogenic tissue that signals for repair of cardiac tissue – the fact that donated MSCs expressed cTnT indicates that they had in fact begun processes coordinate with differentiating into cardiogenic tissue since they were engaged in specific cardio reparative actions and signaling (Kilickap, et. al. 2005). lncRNA-Bvht increased cTnT. See Figure 2.
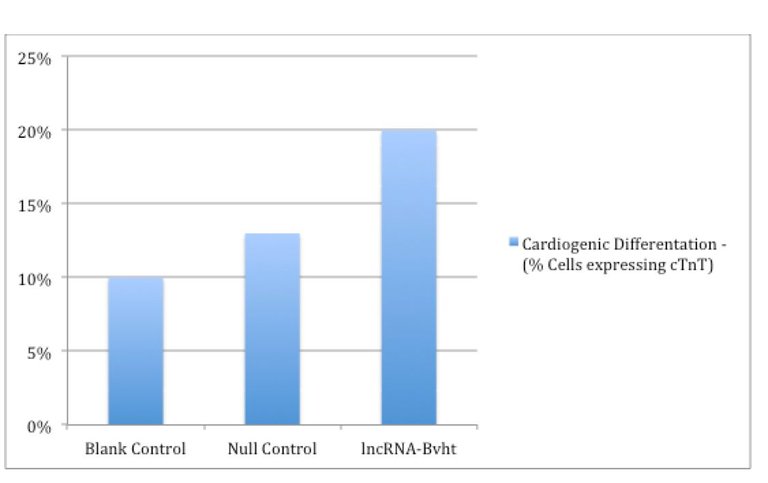
Figure 2: Increased Cardiogenic Differentiation Post lncRNA-Bvt In Rodents
The improvement in cardiogenic differentiation was significant, with the number of differentiated cells approximately doubling relative to blank control, i.e., up to 20% with the application of lncRNA-Bvht transfection (Hou, et. al., 2017).
iii. FURTHER DISCUSSION AND A HYPOTHESIS WORTH TESTING
The two studies principally relied upon here for data lend support to the hypothesis that in humans the use of either of the two RNAs individually (either mRNA-133a or lncRNA-Bvht) could lead to an increase in survivability or differentiation respectively. Again, there have been no studies on humans and these studies cited both involved rodents (i.e., rats and mice).
Based on this evidence, it is contended here that a next logical step would be to test the hypothesis that the simultaneous use of both mRNA-133a mimic and lncRNA-Bvht on a donated population of MSCs, together and in conjunction, could result in significant increases and/or synergistic increases in the total number of cardiogenic tissue differentiated cells beyond the use of either of these RNAs alone in humans.
III. CONCLUSIONS AND FUTURE RAMIFICATIONS
Initially, it was recognized that though stem cells including MSCs provided healing and regenerative benefits through signaling, for the most part they did not survive and very few actually differentiated into cardiogenic tissue. Engineering MSCs with RNA has been extremely promising in that they have been shown in rodent models to increase survivability and differentiation. The ramifications of these data sets indicate that in the near future human stem cells may also be engineered and optimized so that human cardiac tissue will not only be signaled to repair itself by the donated MSCs, but will also receive new young cells that will differentiate, replenish, and replace damaged tissue. Using combinations of RNA to engineer specific affects including survivability and differentiation into specific tissue types represents the current frontier in stem cell regenerative medicine and this paper suggests that mRNA-133a and lncRNA-Bvht are a logical combination that should be tested in conjunction based on current data.
It is also worth noting that scientists are studying a variety of ways to alter and optimize stem cells to increase their ability to effectively regenerate cardiac tissues. One avenue of concern that must be acknowledged is that tumor and cancer cells have in some studies been shown to recruit donated naïve MSCs which then in turn exhibited tumor and cancer like properties since they tend to emulate the tissues they home to – i.e., the plasticity exhibited by MSCs is a two edged sword that must be controlled by engineering (Gwendel & Paula, 2016). Though it exceeds the scope of this paper’s focus on regeneration of cardiac tissue, it is important to note that people with tumors and/or cancer are at specific risk and ongoing research is developing engineering means to avoid MSCs from exacerbating people who have existing tumors and cancer – whether in regression or not (Gwendel & Paula, 2016).
Improving the effectiveness of stem cells has included providing physical and environmental challenges in culture including preconditioning through hypoxia and exposure and treatment with other countless opportunities in RNAs and various peptides, which are implicated in cell metabolism and avoidance of apoptosis (Sarkissian, et. al., 2017). The benefits of these approaches are being established and it is logical to assume that these methods could also be used synergistically and in conjunction with RNA engineering to achieve even better therapeutic results in the future leading to a solution.
REFERENCE LIST
Airas, L., Niemelä, J., Salmi, M., Puurunen, T., Smith, D.J., & Jalkanen, S.
(1997) Differential Regulation and Function of CD73, a Glycosyl-Phosphatidylinositol–linked 70-kD Adhesion Molecule, on Lymphocytes and Endothelial Cells. J Cell Biol. Jan 27; 136(2): 421–431.
Politi, P., Piccinelli, M., Fusar-Poli, P., Klersy, C., Campana, C., Goggi, C., Viganò, M., Barale, F. (2004) Ten years of "extended" life: quality of life among heart transplantation survivors. Transplantation, July 27; 78 (2); 257-63
Kilickap, S., Barista, I., Akgul, E., Aytemir, K., Aksoyek, S., Aksoy, S., Celik, I., Kes, S., Tekuzman, G. (2005) cTnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol. May;16(5):798-804. Epub 2005 Mar 17.
Abdallah, B.M., & Kassem, M. (2008) Human mesenchymal stem cells: from basic biology to clinical applications. Gene Therapy, 15, 109–116.
Mohsin, S., Siddiqi, S., Collins, B., & Sussman, M.A. (2011) Empowering Adult Stem Cells for Mycardial Regeneration. Circ Res., 109(12): 1415–1428. doi:10.1161/CIRCRESAHA.111.243071
Boxall, S.A., & Jones, E. (2012) Markers for Characterization of Bone Marrow Multipotential Stromal Cells. Stem Cells International, Volume 2012, Article ID 975871, 12 pages http://dx.doi.org/10.1155/2012/975871
Law, S., & Chaudhuri. S. (2013) Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. AM J Stem Cell, 2(1):22-38.
Dakhlallah, D., Zhang, J., Yu, L., Marsh, C. B., Angelos, Mark G., & Khan, M. (2015). MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J Cardiovasc Pharmacol, 65(3): 241–251. doi:10.1097/FJC.0000000000000183
Karantalis, V., & Hare, J.M. (2015) Use of Mesenchymal Stem Cells for Therapy of Cardiac Disease. Circ Res., 10; 116(8): 1413–1430. doi:10.1161/CIRCRESAHA.116.303614.
Premer, C., Blum, A., Bellio, M.A., Schulman, I.H., Hurwitz, B.E., Parker, M., Dermarkarian, C. R., DiFede, D. L., Balkan, W., Khan, A., Hare, J. M. (2015) Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine, 2, 467–475.
Fang, Z., Yin, X., Wang, J., Tian N.A., AO, Q., Gu, Y., & Liu, Y. (2016) Functional characterization of human umbilical cord-derived mesenchymal stem cells for treatment of systolic heart failure. Experimental and Therapeutic Medicine. Experimental and Therapeutic Medicine, 12; 3328-3332. DOI: 10.3892/etm.2016.3748
Gwendal, L. & Paula, Y., L. (2016) Recent discoveries concerning the tumor – mesenchymal stem cell interactions. Biochimica et Biophysica Acta (BBC) – Reviews on Cancer, Vol.1866, Issue 2, December 290-299. http://doi.org/10.1016/j.bbcan.2016.10.004
Singh,V. K., Saini, A., Kalsan, M., Kumar, N., & Chandra, R. (2016). Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front Cell Dev Biol., 4: 134. doi: 10.3389/fcell.2016.0013
Wang, L., Zhang, H., Guan, L., Zhao, S., Gu, Z., Wei, H., Gao, Z., Wang, F., Yang, F., Lou, L., Li, Y., Wang, L., Liu, W., & Gao, C., (2016) Mesenchymal stem cells provide prophylaxis against acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: A meta-analysis of animal models. Oncotarget. Sep 20; 7(38): 61764–61774. doi: 10.18632/oncotarget.11238
Nichols, H. (2017) The top leading causes of death in the United States. Article Public Health, Medical News Today. http://www.medicalnewstoday.com/articles/282929.php
Hou, J., Long, H., Zhou, C., Zheng, S., Wu, H., Guo, T., Wu, Q., Zhong, T., & Wang, T. (2017) Long noncoding RNA Braveheart promotes cardiogenic differentiation of mesenchymal stem cells in vitro. Stem Cell Research & Therapy, 8:4. DOI 10.1186/s13287-016-0454-5
Sarkissian, S. D., Lévesque, T., & Noiseux, N. (2017) Optimizing stem cells for cardiac repair: Current status and new frontiers in regenerative cardiology. World J Stem Cells, 9(1): 9-25. DOI: 10.4252/wjsc.v9.i1.9
Congratulations @sunnydays! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPThanks I guess?