Recently, I have been having very good reads on a lot on science articles, (want to know where? Read till the end) and believe me, I am beginning to question the love I have for the field of art I am in. Art is beautiful, science is brilliant. Precise knowledge on these two fields of endeavour makes the perfect blend. Like I said earlier on, I have been reading, more like I have started from the basics. While I was studying in the arts, much of what I heard the science folks discuss were the likes of oxygen, hydrogen, carbon etc.. I really didn't have precise knowledge about these elements, but then today I believe say a thing or two about them -- I will try that out now.
The element, carbon has the atomic number 6 and symbol C, it is a very vital element of human life. Carbon exist in almost everything available to human; even the simple things of life. I believe this is a very useful piece of information on the history of carbon from britanicca:
Carbon as an element was discovered by the first person to handle charcoal from fire. Thus, together with sulfur, iron, tin, lead, copper, mercury, silver, and gold, carbon was one of the small group of elements well known in the ancient world.
The element carbon has been around for a relatively long period of time as its discovery is somewhat lost in history. The element which was known to pre-historic humans in the form of charcoal is found virtually everywhere around us.. Especially in materials we make use of on a daily basis such as plastics, perfumes, polish, our jewelries, petroleum to mention a few. You would readily find carbon in different forms, let's take a look:
- In the form of carbon dioxide - it is used in the production of fire extinguishes, and drinks, the fuzzy kinds.
- In the form of freon, it is used in cooling devices as well as cooling systems
- And in metallurgy, carbon in the form of carbon monoxide is used as a reduction agent to derive some other elements as well as compounds.

Aside being used for material things, carbon is also of importance in the human body. It happens to be the very basis of proteins, fats, as well as nucleic acids found in the body. Ultimately, in the physiology of the body, it has a very important role. Without carbon dioxide, we would not be able to breath, as carbon, resident in carbon dioxide, is what makes it possible for use to do. A high level of carbon dioxide could choke on one's health, so it's important to maintain a normal balance of it in the body. If you look at it quite closely, you'd find out that carbon is also a building block for quite a lot complex and important life processes, the element carbon in our body is what's responsible for the collection of diverse atoms and thus gets them to function, thereby aiding growth. Worthy of note is the fact that the human body is made up of about eighteen percent (18%) of carbon source can you beat that!!

Well, having said that, you probably have heard of a couple of other precious elements such as carbon, but have you ever heard of graphene?! Well, if you haven’t, am guessing you should grab a pack of chilled juice, as we take a ride to learn about one of the world's most amazing element existing -- the miracle material GRAPHENE!!
What is Graphene?
Any of the element could exist in so many different forms and yet still remain in it's same physical state, these different forms of an element are referred to as allotropes. Each allotrope differ from others but only in a slight structural variations. Lets take for instance, carbon (which was introduced earlier), it's known to have just a few allotropes, which are: fullerene, graphite and of course the very popular diamond, amongst others.
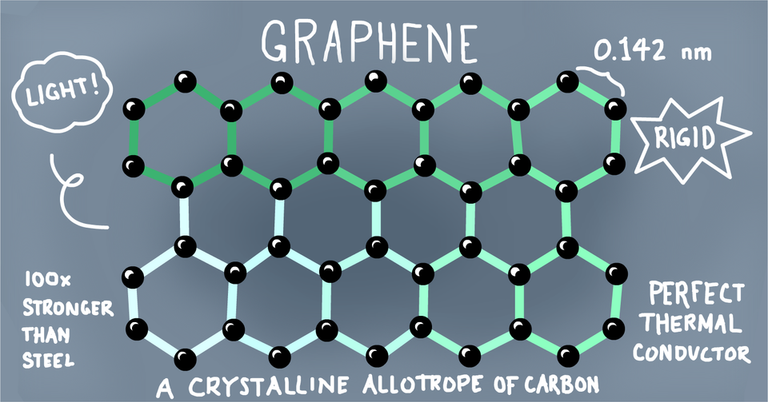
Graphene is another allotrope of carbon, although it's been less popular for a while, and is available in form of a two-dimensional lattice. A little fifth grader insight into this makes it a whole lot easier.. look at it this way; pencil lead is made from graphite, this graphite are simply attached thin layers of carbon. I. e lead is made up of thin layers of carbon above each other. These this layers are called graphene, put differently, they are just single layered carbon atoms which are arranged and forms a honeycomb pattern... this should be much easier to comprehend.
Graphene became popular in 2004, when two scientist from the University of Manchester in the persons of Prof Andre Geim and Prof Kustya Novoselov were able to successfully isolate a single layer crystaline carbon from a bulk of graphite (cost effectively); these two dimensional single layer of carbon is what we now refer to as graphene. The singular feet became the discovery of the decade and won them the 2010 Nobel Prize in Physics. The two dimensional structure and strong ladder of the graphene provides it with properties not seen in any other element known to man; amongst this includes it's strength and stiffness, thinness and lightness, conductor of heat, conductor of electricity, impermeable etc.. Having mentioned all these, I just thought about it, how do we derive graphene given that it's a allotrope of carbon?
How about we make some GRAPHENE?
Unlike a wide range of other elements, graphene appears to be quite easy to make, even a fifth grader could do this successfully, although this would also come in small quantity which could be used for laboratory testing sorta. So let's get down to it, this simple process of making graphene is called mechanical exfoliation and it involves the use of a pencil and a sticky tape. Firstly, get a pencil, try sketching on a piece of paper, place your sticky tape on it and pull it off, you should have gotten a layer of graphite which is made up of multiple layers of carbon atoms. Go over the process of placing your sticky tape and removing it over and over again, till you are able to end up with carbon with just a single layer -- volla that's your graphene!!
You could try out loading up a super-precise atomic force microscope with a little piece of graphite and then rub it with apt precision on something in order to make the single layers of graphene come off.
These two approaches to making graphene as mentioned earlier would only produce little quantities which could be used for laboratory testing but making large quantities of graphene would require the use of a more complex method called chemical vapor deposition (CVD), a process you can read on here. Let's conclude this whole discussion with how this super element would power the future.
The future of GRAPHENE!!
The graphene material appears to be reasonably very remarkable in so many different ways, it's discovery has overtime inspired researchers and scientists alike to think of a wide range of potential applications for these material, although still in its laboratory testing state, let's look through at what they have been able to come up with:
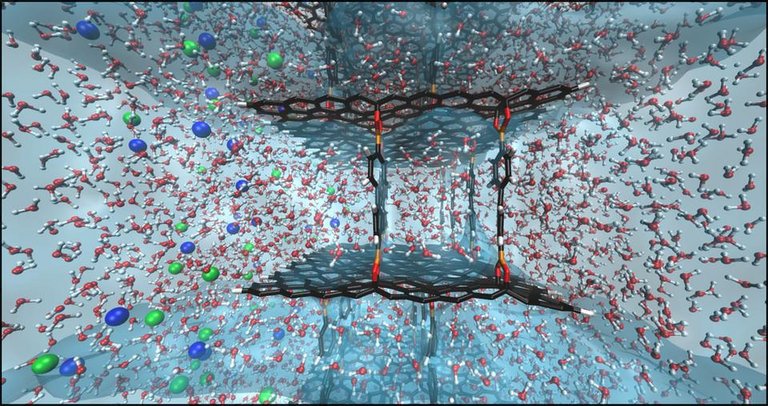
Graphene and water filtration
Ever heard of Mahatma Gandhi? He survived 21days without food but can we go that long without water? Definitely not. Some scientists have said a week is the maximum; that shows how much we all need water. Well not just any water, pure form of water. Consumption of clean water and maintenance of its supply is of utmost importance in the world, considering the continuous increase in the world population, but this is somewhat unfortunate as the availability of fresh water for human consumption in recent times has been somewhat limited. Thankfully, graphene could be used to fix this.
Graphene's nature has made it impervious. This means it is almost entirely impossible for any form of gas or liquid to pass through it, but surprisingly water is an exception. This surprising fact coupled with its ability to act as a water filter for the toxins that might be present in it, has made graphene an effective material to be used in leaving water absolutely free of contaminants. The adoption of this would be a great solution in fixing the problem of water filtration, improving water filtration. A graphene filter called Perforene was recently developed by a scientist named Lockheed Martin, his company believes this to be a revolution in the desalination process of water filtration.
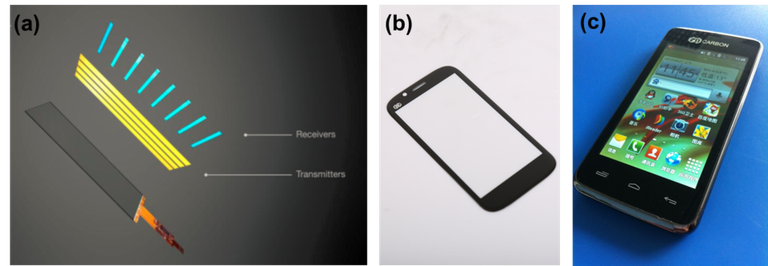
Graphene and electronics
As early stated, graphene gained it's popularity in 2014. That same year, it's global marked was reported to have achieve an all time high of nine million dollars ($9,000,000). Most of this could be attributed to its use in the production of electronics.
Graphene's flexibility and highly powerful electrical properties has made it a somewhat flawless material for the manufacturing of portable electronics. All thanks to its flexibility, the production of miniaturized machines as well as sensors is possible. In turn, making it an excellent material for the creation of smartphones and tablets which could be more durable. The market for wearable electronics is increasing, electronics made with graphene could be designed in a better way; fitting perfectly around the limbs and also bend accurately to accommodate exercise conditions. Can we all agree that the adoption of graphene for the electronics could make things better and easier?
Graphene and solar cells or photovoltaics
Factually, the sun (sunlight) has the capacity to produce about eleven thousand watts per meter square of energy (11,000 watt/m2), but not all of that could be converted into electricity. The conversion efficiency peak stands at thirty three point seven percent (33.7%), even after four tons of the recommended band gap has been utilized, this is known as the Shockley Queisser limit.
Scientists have recently been staching up thin layers of solar panels on each other such as multi-junction solar cells in order to gain the ability of passing that limit. The result of these has been a product which isn't really economically reliable for domestic users.
The conversion limit can be reduced by another factor: the presence of mesh wires or charge carriers which obviously has to be placed on solar cells, however they are known to prevent the light from passing through them, as a portion of light is usually lost in there via copper or aluminum viles on the front of the panel. Bringing graphene into the big picture, it's electrical conductivity and optical transparency makes it ideal for use in solar cell. In this case graphene could be adopted and used as the charge carrying matrix which is placed on top of the solar cells. We working with reports right? Well, reports have it that graphene based solar cells have already been able to attain an efficiency of 15.6% which is known to be very high for flexible solar cells. More researches on graphene's use in solar cells are already ongoing, and the efficiency of these cells are still on the increase. Hopefully and in due time, scientist will come up with cheaper and more powerful graphene cells which ultimate result would be a massive surge in renewable energy.
Graphene and batteries
It is a little obvious that one of the biggest problem of battery technology over time is their energy density. Which mean lithium-ion's battery (popularly known as Li-ion battery) discovery certainly has addressed the energy storage issue to a great extent, even though its use on a utility scale has still not taken off.
Addition of graphene to this could increase it's energy storage capacity significantly.
Li-ion battery has a problem (which is possible inherent) in its design. For more power to be drawn, the battery's charge scaling channels has to be increased and this reduces the space for its charge storage. I read an instance that is quite synonymous to an aeroplane.
The increase in the number of doors a plane has would allow passengers to board and highlight safely and easily and has much as that is a good idea it would ultimately reduce the number of passengers the aeroplane can board at a time.
Graphene's conductive characteristics would allow it to be used as a charge carrier, occupy less space unlike other materials and solving the problem stated in the above illustration. As these would ballow more space for charge storage in the battery. So it's use would mean that high powered batteries could also be high energy batteries. I am here with imaginations of my phone being powered by graphene. The smile on my face is wide....
Some other applications of GRAPHENE
Semiconductors - It's high conductivity rate would allow graphehe's use in semiconductors to a great extent, eventually bringing about an increase in the speed at which information is passed.
Lubricants - Graphene would definitely be able to deal with shear forces much better than any other element considering its high strength and atomicly smooth structure.
Sensory aid (in prosthetic limbs)
There are more ways graphene can be used but I am stopping here.
Looking at the somewhat endless list of the strengths which graphene possesses, we'd expect to see it all over us by now. But the case is different, Why? At this point, it's quite expedient that we know that while the production or making of graphene in little quantities for laboratory use could be cost friendly, it is really very expensive to mass produce it. This ultimately limits its use in products or researches which require it's use in large quantities. Additionally, just as with other materials, when mass production kicks off, there are slight possibilities that some flaws or fissures would pop up. We should know that irrespective of how amazing a discovery in science is, finances would always be a major determinant of its success. The future is definitely graphene (at least would be built on graphene) notwithstanding the current challenges and limitations graphene is facing, researchers are still trying out all sorts of uses for graphene.
@gidionline
References
Nanowerk - What is graphene
Britannica - Structure of carbon allotropes
Nobel Prize - Nobel prizes physics laureates
Independent -What is the human body made of
Learning English - Graphene: the material of the 21st century





Pretty interesting article about graphene. These smaller bits are found in most substances in nature and life. Heard they're tougher than diamonds to an extent.
Thanks buddy @pangoli
Yeah, these allotrope of carbon is definitely small but mighty and have so many applications to life -- relatively hard, but compared to diamond, well relatively
Thanks for dropping by buddy @pangoli
Seeing your title, it caught my attention almost immediately... Supporting you stand, I think graphene actually had the potential to power the future, but then just as you have rightly pointed out here:
Getting this miracle material in large quantities might take months, years and even decades before its fully achieved.
A quite lengthy but yet educative science article. @gidionline
Thank you @austinejay.
Graphene is a material of the future
I see you had quite an interesting read on here, endeavour to drop by again.
Future could be tomorrow. Haha @gidionline
Lol.
You got this right @traviz
I sure will @gidionline
This graphene material, it's called a miracle material. I guess Samsung has already adopted it in the manufacturing of some of their devices.
Good read @gidionline
Yeah, I read somewhere and it was dubbed the miracle material of the furure
...well, about Samsung, I think so.
Thanks for dropping by @ traviz
I'm going to do this exactly, I've heard of graphene but I've never thought of it as this useful, guess I was doing the wrong thing all these while... Lol
Kudos man, this is awesome.
Thank you @joelagbo
It would be nice if you could do your research also..
Write an article.. so I can learn from you also.
Thanks for dropping by. Appreciate.
Sure, I'll get back to you on this.
Good stuff, I am also a believer in graphene and in other new materials.
I think that the future will bring many more materials, I have my eye on the carbon nanotubes and nanomesh (or how is it called).
Pretty good job of describing and looks original and nice, at least to a first impression and check!
Great job!
Thanks @alexdory
That makes two of us
Definitely, the future holds a lot of elements in store for us, soon we would be hearing of so many others. Read a little about the carbon nanotubes while researching on graphene, cool stuff.
Thanks for dropping by @alexdory, we going to the moon together.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by gidionline from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
#Bigwaves
#Onequality