Rust, Mechanism of Rusting and Its preventive measure
Iron is one of the most important elements in our daily life. Most of the equipment are made up of iron. But, eventually a reddish brown layer gets deposited in the iron equipment. It corrodes the iron and makes the equipment weak. In this article, I am going to write about scientific theory behind this phenomena and prevention.

(pixabay.com)
Iron has no action with dry air but reacts with air in presence of moisture to form reddish brown solid which is called rust.
.png)
The process of formation of rust is called rusting.
It is best example of corrosion.
Mechanism of rusting
Among different theories of rusting, Electro chemical theory is the most acceptable theory to explain mechanism of rusting.
According to this theory, the rough surface of iron can hold the atmospheric moisture containing dissolve air and acts as electrochemical cell. In this cell surface of iron, iron acts as anode and as oxidation takes place at anode; iron passes into solution as 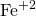 by leaving electrons.
by leaving electrons.

Air dissolved in water acts as cathode and as reduction occurs at cathode; it readily gains electron and forms  .
.
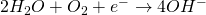
These two respective ions migrates towards each other, combines and forms Fe(OH)2

Ferrous hydroxide so formed readily undergoes oxidation to form 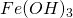 .
.

Ferric hydroxide which is unstable then readily re arranges to give reddish brown solid (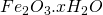 )
)
Hence actual composition of rust is 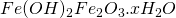 . Impurities present in moisture increases the rate of rusting as it increases the migration of ions.
. Impurities present in moisture increases the rate of rusting as it increases the migration of ions.
Preventive measures of rusting
The surface of iron can be coated by paint, oil, grease, etc. The car in automobile is not just to make it attractive. The paint in your automobile prevents rusting in it. The machining tools and machines are constantly provided with grease for lubrication and to prevent rusting.
The surface of iron can be coated by more electropositive metals called zinc, which is called galvanization process. In this process, the zinc gets corroded instead of iron.
Iron can be made passive by dipping iron with certain chemicals like conc. HNO3, chronic acid, etc.
By sacrificial anodic protection:
In this method iron is coupled with electropositive metal which acts as anode. As oxidation takes place at anode more electropositive metals undergoes oxidation and prevent iron from corrosion.Iron utensils shouldn’t pose deep corners and peaks. If it peaks and corner is to be made it should not be cleaned up regularly.
Two metals with electropositive difference shouldn’t be couple together. If they need to be coupled insulator should be used.
References:
Conceptual Chemistry; SK Jain
Foundations of Chemistry; Moti Kaji Sthapit
You got a 1.87% upvote from @upmyvote courtesy of @bikkichhantyal!
If you believe this post is spam or abuse, please report it to our Discord #abuse channel.
If you want to support our Curation Digest or our Spam & Abuse prevention efforts, please vote @themarkymark as witness.