Introduction
Radiation can be found all around us, and has been in existence even right before the formation of the earth. What comes to many people’s mind when they hear the word “Radiation” is a perfect misconception of the word itself. Many members of the public have a misinformed view that radiation has no place in a safe and healthy world. Many even think radiation is the emission of rays or particles that destroys anything that comes their way. Not knowing that if it has been what people think it to be then all creatures would have been in extinct. Radiation is a natural feature of the environment, meaning that many things around us are potentially radioactive. To shock you, 80% of radiations are natural while the rest are initiated by man. While radiation is a term that most people have heard, the basic facts about radiation are much less familiar. Then what is radiation? What causes radiation? What are the types of radiation? Is radiation harmful? What are the risk of getting harmed?...
What is Radiation?
Imagine a big ball made of magnets that’s spinning really fast. Sometimes a few pieces of the magnet will shoot out and hit the wall. That’s kind of what radiation is like.
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
- Electromagnetic Radiation, such as radio waves, microwaves, visible light, x-rays, and gamma radiation (γ)
- Particle Radiation, such as alpha radiation (α), beta radiation (β), and neutron radiation (particles of non-zero rest energy)
- Acoustic Radiation, such as ultrasound, sound, and seismic waves (dependent on a physical transmission medium)
- Gravitational Radiation, a radiation that takes the form of gravitational waves, or ripples in the curvature of spacetime.
The word radiation arises from the phenomenon of waves radiating (i.e., traveling outward in all directions) from a source. Radiation is energy travelling through space. Radiation can come from as far away as outer space and from as near as the ground that we are standing on. Radiation sources are naturally all around us. Sunshine is one of the most familiar forms of radiation.
Atomic Basis
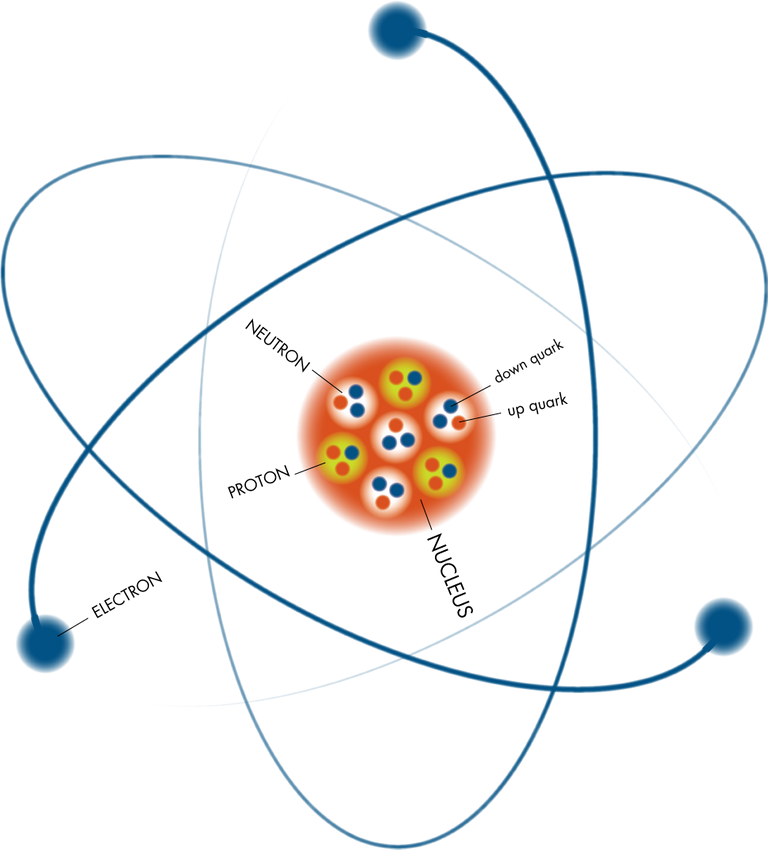
All matter is composed of atoms. Atoms are made up of various parts; the nucleus which contains minute particles called protons and neutrons, and the atom's outer shell which contains other particles called electrons.
The nucleus carries a positive electrical charge, while the electrons carry a negative electrical charge. These charges within the atom work toward a strong and stable balance by maintaining equal number of opposite charge and getting rid of excess atomic energy. In that process, unstable nuclei may emit a quantity of energy, and this spontaneous emission is what we call radiation.
Radiation comes from the nuclei of atoms, the basic building blocks of matter. Most atoms are stable, but certain atoms change or disintegrate into totally new atoms. These kinds of atoms are said to be 'unstable' or 'radioactive'. An unstable atom has excess internal energy, with the result that the nucleus can undergo a spontaneous change. This is called 'radioactive decay'. An unstable nucleus emits excess energy as radiation in the form of gamma rays or fast-moving sub-atomic particles.
History and Discovery
Radiation got its start in 1895 with Wilhelm Röntgen. In the process of conducting various experiments in applying currents to different vacuum tubes, he discovered that, despite covering one in a screen to block light, there seemed to be rays penetrating through to react with a barium solution on a screen he’d placed nearby. After several experiments, including taking the first photo with the new rays, he named them “X-Rays” temporarily as a designation of something unknown.
This discovery was followed in 1896 by Henri Becquerel’s discovery that uranium salts gave off similar rays naturally. Becquerel, a French scientist while working with phosphorescent materials, observed that a crystal of uranium salt spontaneously emitted radiation which could penetrate through opaque materials to affect a photographic plate. Becquerel concluded that the uranium atoms were responsible for the emission of this unknown radiation which he called "Becquerel Rays".
Image source
Becquerel’s doctoral student, Marie Curie, named this phenomenon: radioactivity. Marie and Pierre Curie’s study of radioactivity and their research on Becquerel's rays led them to the discovery of both radium and polonium. Their exploration of radium launched an era of using radiation (radium) for the treatment of cancer, which could be seen as the first peaceful use of nuclear energy and the start of modern nuclear medicine.
Types of radiation
Radiation is often categorized as either ionizing or non-ionizing depending on the energy of the radiated particles that is the capability to knock electrons off atoms that it interacts with.
Ionizing radiation:
Ionizing radiation carries more than 10 eV, which is enough to ionize atoms and molecules, and break chemical bonds. It is dangerous and has enough energy to damage cells, DNA and cause cell mutation. Ionizing radiation is everywhere including in minerals in the soil, and a common source is radioactive materials that emit Alpha (α), Beta (β), or Gamma (γ) radiation, consisting of helium nuclei, electrons or positrons, and photons, respectively. Ionization occurs when an electron is stripped from an electron shell of the atom, leaving the atom with a net positive charge. Because living cells and, more importantly, the DNA in those cells can be damaged by this ionization, exposure to ionizing radiation is considered to increase the risk of cancer. Ionizing radiation includes: ultraviolet radiation, Gamma radiation, Alpha radiation, Beta radiation, Neutron radiation, X-rays, and Cosmic radiation.
Non-ionizing radiation:
Non-ionizing radiation being a less energetic radiation is considered harmful only to the extent of the amount of heat energy it transfers to whatever it hits. This type of radiation deposits energy in the materials through which it passes, but it does not have sufficient energy to break molecular bonds or remove electrons from atoms. Non-ionizing radiation includes visible light, heat, radar, microwaves, and radio waves
Causes of radiation
Radiation can be experience from either a Natural or Man-made source
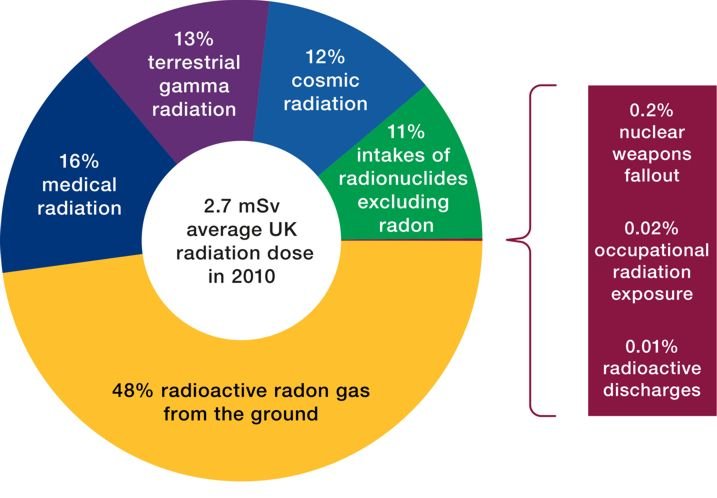
Naturally Occurring (Background) Radiation
Background radiation is that ionizing radiation which is naturally and inevitably present in our environment. We can be exposed to radiation naturally from Mineral materials or elements (like Radon, thorium and potassium) in the earth crust and also radiation from space (cosmic).
People living in granite areas or on mineralized sands receive more terrestrial radiation than others, while people living or working at high altitudes (elevations) receive more cosmic radiation. A lot of our natural exposure is due to radon, a gas which seeps from the Earth's crust and is present in the air we breathe.
Man-made Radiation
Exposure to radiation can also be from man use of radioactive materials. The medical field makes use of radiation during procedures like x-rays and CT scans to diagnose injuries and diseases in patients. Drugs with radioactive material attached, known as radiopharmaceuticals, and radiation therapy (use radiation to treat patients) are also effective clinical applications.
Industries use radiation in a variety of ways. For example, radiation is applied in checking for weak points in metal parts and welds before products are sold. Other examples include irradiators and nuclear research and power reactors. All these activities create avenue for exposure.
Unit of measure
Radiation dose is a measurement of how much energy is deposited into a material from a source of radiation. Radiation exposure is measured primarily in Rem (in the US) and the Sievert (SI unit), and is a measure of the radioactive dose absorbed relative to its possible health effects on the body. This is called the “equivalent dose,”
Radiation exposure can be Acute or Chronic. An Acute exposure is a dose of radiation received all at once; examples include doses involved in cancer therapy. While a Chronic exposure is low levels of exposure over a long period of time; examples could be exposure from high levels of radon in a basement, or someone living at high altitudes.
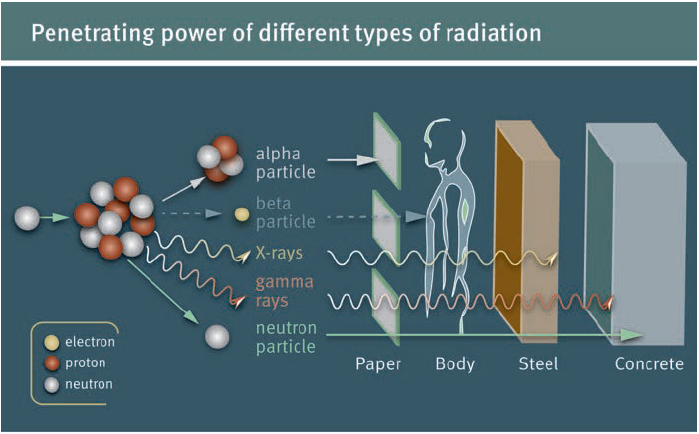
Image source
Uses and Applications of Radiation
- Medicine: Radiation and radioactive substances are used for diagnosis, treatment, and research in the medical field. They are used in x-rays to obtain detailed imaging of anatomical structures. This property of X-rays enables doctors to find broken bones and to locate cancers, diseases or abnormal structures in the body. Radiation is also useful in the pharmaceutical industry for preparation of radiopharmaceuticals.
- Science: Researchers use radioactive atoms to determine the age of materials that were once part of a living organism. The age of such materials can be estimated by measuring the amount of radioactive carbon they contain in a process called radiocarbon dating.
- Communication: All modern communication systems use forms of electromagnetic radiation. Information are being transmitted through electromagnetic waves, like radio waves, microwaves by creating variations in the intensity of variations to represent change in information (sound, pictures etc)
Conclusion
“There is no safe level of exposure and there is no dose of (ionizing) radiation so low that the risk of a malignancy is zero”
--Dr. Karl Morgan
References and further Reading
- https://en.wikipedia.org/wiki/Radiation
- http://www.world-nuclear.org/nuclear-basics/what-is-radiation.aspx
- https://en.wikipedia.org/wiki/Radionuclide
- https://www.epa.gov/sites/production/files/2015-05/documents/402-k-10-008.pdf
- https://www.fs-ev.org/fileadmin/user_upload/89_News/Oeff.-Arbeit/Radiation_Effects_and_sources-2016.pdf
- https://whatisnuclear.com/math-behind-radioactive-decay.html


It look like you have copied directly from this source
You wrote this:
In the book it says this:
Tell me you didn't copy most of this article from different sources.....
No I didn't ... although, I don't know what explanation to give for this, but 1; I didn't used the book as a resource material and 2; all materials and pdf used as references are acknowledged in the reference section.
There is a difference between learning from material, and giving a reference as oppose to taking material and copying it.
I found a few examples here of copying, no ways it's a coincidence. It's also a bit strange that your first post on steemit is steemstem post and written in an accepted format.
Can you explain this please?
sourcewell, for me being able to write in a steemtem accepted format, is as a result an orientation i had from a brother of mine @okunlolayk who introduced steemit to me and also written source like this;
Very interesting mate
Congratulations @bayouz! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your Board of Honor.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPCongratulations @bayouz! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @bayouz! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @bayouz! You received a personal award!
Click here to view your Board
Congratulations @bayouz! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!