These posts are not for foraging. They are intended for entertainment and intellectual satisfaction only. These posts are not a field guide nor comprehensive in any way - their accuracy is not assured in any way. Do not eat wild mushrooms unless you are a professional, have substantial professional assistance or have a wealth of personal experience with a specific species. Do not make any foraging decisions based on these posts. To do so could be dangerous or life threatening.
This post makes no reference to edibility or toxicity
Update!
After a bunch of sleuthing I managed to find two awesome and comprehensive online resources for Myxomycete species and I believe i have identified this species!
It fits the bill remarkably well for Licea variabilis. Sessile, dark gray/black, with brown, round spores 12-13 microns across!
I couldn't be more pleased!! That would make this the first successfully grrown and ID'd slime mold in my little lab. New sources added.

These strange fellas appeared overnight one day.
I was scanning this particular piece of wood quite frequently under the microscope for the photographic mini-series I've been doing called "Mapping A Tiny World". As a result, I was checking it every day in effect, toying around with different angles and lighting.
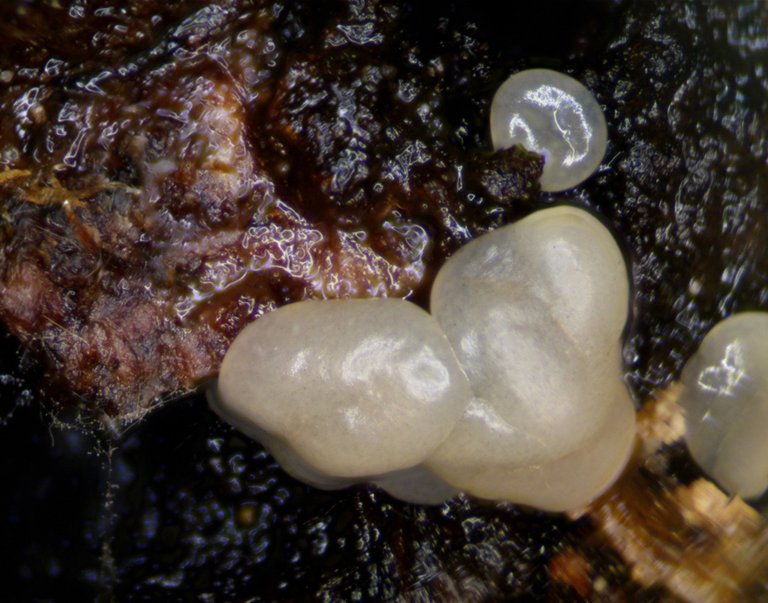
So, imagine my surprise when, over the course of a single night, I woke up and found these shiny, jelly masses adhering all over my little piece of bark. I thought I had finally hit the slime mold jackpot! This looked like it could possibly be a plasmodium... a bit large and robust and sudden to be sure, but, why not?!
So, I decided to hedge my bets and try to take a time lapse. Maybe, just maybe, I would capture something awesome

"Taking a time lapse" turned out to be less than straightforward.
On the one hand, the AMscope software makes taking a time lapse video about as easy as possible. In this case I set the program to take 1 picture every minute for 8 hours. I ended up needing to turn it off in the middle of the night, about 5 hours in, because the light was keeping me and my wife awake. This is all happening in my tiny bedroom laboratory after all.
Unfortunately, time lapse photographing a slime mold, or even a growing fungus, is more complicated than simply setting up the camera and leaving it. These organisms can be extremely sensitive to both light and moisture content. At the same time, my camera requires a certain amount of light, even at the highest exposure, in order to capture anything.
So, I spent an hour working out a perfect balance. First, I knew I could not remove the cover of the petri dish. To do so overnight would dry the entire ecosystem out, probably fatally. However, second, I found that if I left the dish cap on and allowed for direct LED illumination, even at low levels, it caused a slight increase in temperature, which in turn caused a TON of condensation.
I needed to get as little light as possible on the specimen while maintaining the ability to visualize what was happening. Above you can see my extremely make shift approach. The petri dish is ensconced in towels to help maintain an even temperature, as my bedroom can get drafty at night. Meanwhile, the light is turned down to its lowest setting, and I've inserted small slivers of white paper to help reduce the direct light even further.
The results of my efforts are below. The video encompasses about 4 to 5 hours at 24 frames per second. You will see that my light/heat tricks worked, as the condensation slowly recedes as the video progresses. You will also see that, although it is not plasmodial movement, I do in fact capture something pretty strange.
Keep your eye on the fruiting bodies at the bottom of the video.
What? FUNGAL BLOB is evolving!
That is not a trick of lighting - the lighting stayed even all night long - the fruiting bodies matured overnight!

Here's a macro shot of the final product!
How crazy is that?! Overnight, the strange organisms, which had only appeared less than 24hours earlier, morphed super quickly into a totally different look. The almost clear fruiting body structures became completely opaque, and differentiated between a thin outer skin and a brown, fig newton colored and textured spore mass.

Moreover, the transformation happened uniformly across all of the fruiting bodies on the piece of bark. There were about 8 of them altogether, with the larger, measurable by calipers, coming out at 1.9mm at its widest. Each cap was fairly well dispersed across the bark, and yet all of the caps appeared and matured at nearly identical rates.
Finally, and this is the really crazy thing, the bulk of the change occurred within about a single hour. It's almost like a chemical reaction - it took a little while to get started, but once it got rolling, the transformation was quick and inexorable.
I needed to get an interior sample to examine under the compound microscope - so I thought it would be helpful and interesting to capture some footage of that process. You can see the Fig Newton like color and texture of the spore mass. I'll make a reverse .gif of this as well, cause I think that would be oddly satisfying.
Once the sample was taken, I placed it under the compound scope.
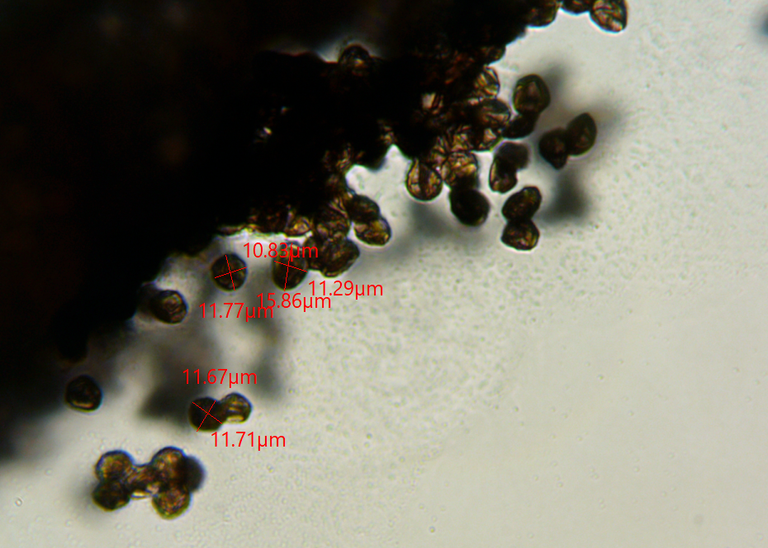
These spores looked vaguely circular, but with ill defined edges.
These are from a sample I took the morning after the time lapse. I waited a couple of more days and then returned to take a new sample, thinking that something might have changed. It did.
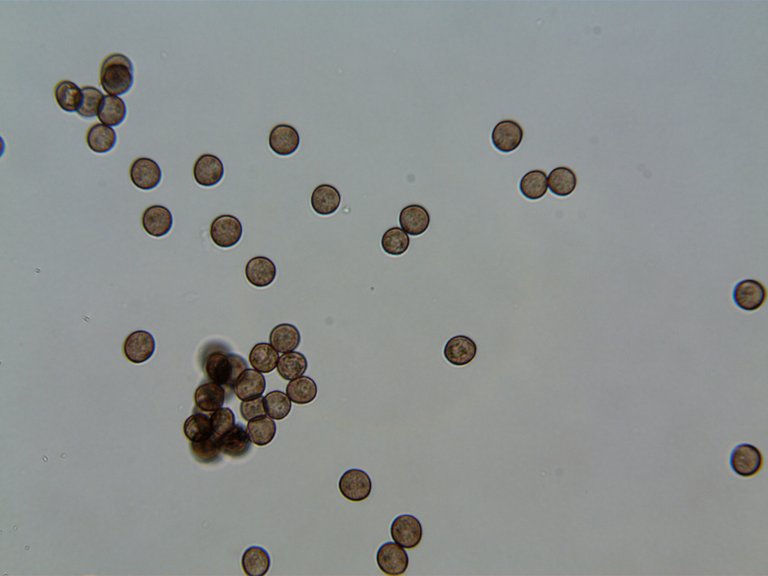
These are the beautiful, round spores taken from a different cap of the same species several days after the transformation.
You can see that the spores are now very clearly defined in their shape and color. It's hard to say without the 100x immersion oil lens, but based on the well defined outer edge of each spore, I would guess that these spores have a fairly thick skin or "Perispore."
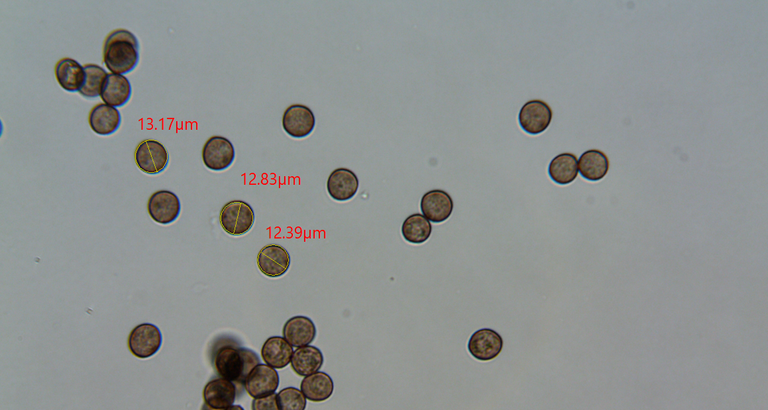
I took measurements of a few and they ranged within roughly 12-13ish microns in diameter.
As always recently, we are left with the burning question - what are we looking at exactly?
I'm afraid we find ourselves in a similar state of ignorance, although not for lack of investigation.
If you take a look at the sources for this post, you'll see that there are a bunch. I run these tiny finds through all of the guides I own, then through all the internet resources I know about, and in this case even through google scholar looking for more comprehensive and obscure keys and scientific papers.

Here, there were several species/genuses that I encountered that seemed plausible at first, but didn't work. The Exidia genus contains some species that look like an OK match on the surface. Take Exidia glandulosa to the left. However, the species, and most of the species in Exidia I could find had very specific, sausage shaped spores, unlike the very round ones present here.

The Daldinia genus also came to mind. However the species I could find all seemed to grow on a totally different scale, in the 1+ cm range, than the species I found here. Now I've heard that moist chamber grown specimens don't always grow to their normal ranges, but this would be a stretch. Plus Daldinia concentrica, to the right, is a Ascomycete. The spores shoot out through the surface of the fruiting body, and they are a different shape overall.
The specimen we have here doesn't appear to be an Ascomycete. I've returned to look at the fruiting bodies several times in the last week or so since they matured and they don't seem to have expelled any spores like an Ascomycete would. Plus, the roundness of the spores seems to militate against the idea that they would be shot out of asci, a structure which I did not see under the compound scope.

The organisms I'm looking at here seem closer to the final product of Lycogala epidendrum, or one of the fungal puff balls. I will keep watching them to see if/how they actually disperse their spores. So far, given the ease with which I damaged them, I think it might be by means of physical agitation. However it could be that they form a dispersal hole in coming days. To be clear, these are NOT L.epidendrum. The spores are too large and the fruiting bodies are morphologically quite different.
Another option up for grabs was a Hypoxylon species. I only considered the genus after finding one in Baroni's field guide. He covers one species, H.fragiforme. However the spores are ellipsoid and the surface of the fruiting body is textured differently.
In the days since I originally pictured the mature specimens, they have darkened more, but so far have not been seen to release their spores, or change their outer texture.
Which leaves us not quite, but almost, without a clue. Well, that's not entirely fair to say. We have a clue - without any precursor plasmodium, I am pretty sure these are fungi and not Myxomycetes. Beyond that, there are just certain types of mushrooms which require more information to identify than I can bring to bear. Indeed maybe more than most can bring to bear.
Consider the research paper cited to below, which gives a fairly comprehensive species list and key for the Daldinia genus.
The paper's authors concede that species identification for the Daldinia genus often requires a scanning electron microscope! Nonetheless, they tried to make a key using morphological traits, as best as they could. What is step one? Place the spores in a KOH solution and see if the super thin outer casing splits open. This means step one is to look for a trait I cannot possibly see with any certainty using my current compound microscope. Which is why an upgrade is in order.
But, I don't think that this process has been unfulfilling or fruitless, even though getting firm identifications has proven near impossible.
I've always envisioned these posts as being an exploratory journal. My hope is to inculcate in others the enthusiasm and wonder I feel when I find this sort of thing - and then engage in a fairly structured process to try and find some answers. I have no illusions that those answers will always be forthcoming - indeed, as I say in my disclaimers, I tend to think I will be in the dark often, or even get things wrong from time to time. But it will never be for a lack of effort. IMO it's much better to admit ignorance and try to rectify it than to pretend to have knowledge.
Especially here on Steemit, I guess I could just pick a random lookalike species and say "This is it." Probablno one would look into it, or debunk me, and it would it make me look like I really know what I'm doing! But it would also be totally intellectually dishonest and amoral. I would much rather be frank about where the borders of my knowledge are and try to expand those borders whenever I can. Because with every failure to find the thing I'm looking for, I learn more about things I never knew existed.
Photos And Videos Are My Own Except:
Microscopic photos were taken using an AmScope SM-4TZ-144A dissecting microscope and an AMScope M150B entry level microscope. The camera lens is also AMScope, MU300.
Information Sources
The bulk of the information in this case was derived from a multi-part posting of these photos and the circumstances of their being taken on several forums involving the study of Fungi and Slime Molds. The information was shared on The Slime Mold Collective, The NYMS Facebook page, and the subreddits /r/mycology and /r/slimemolds. Users from the slime mold collective and reddit both expressed doubt that this was a slime mold and was in fact a fungal mold.
Efforts were made to identify the species using the following books:
[1] Myxomycetes A Hand Book of Slime Molds By Steven L Stephenson and Henry Stempen
[2]The Audubon Society Field Guide To North American Mushrooms by Gary Lincoff, p.382, Exidia
glandulosa.
[3]Mushrooms Demystified By David Arora
[4]Mushrooms of the Northeastern United States and Eastern Canada by Timothy J Baroni, p.563, Hypoxylon fragiforme
[6]Kuo, M. (2007, April). Bulgaria inquinans. Retrieved from the MushroomExpert.Com
[7]Wikipedia on Exidia Glandulosa
[9]Discover life on L.variabilis
[10]slimemol website on L.variabilos
Quite a delicate matter setting up to record the time lapse, they really are so robust in some ways and fragile in others. That in itself was an eye opener for me. It was worth it in the end, all that effort. I really appreciate all the work you put into these posts, and trying to identify all the different species. And in the end it brings up some many questions, to me that's what true learning is about. Great job again @dber
Thanks @trucklife-family - at some point I am going to need to involve myself in some external learning - I don't want to pretend that just auto-didactic exploration is sufficient to increase my skill level ad infinitum. It is a very fulfilling activity, to be sure, and a lot of fun - but it has its limits. However, that's where my efforts in the next few months to get more involved with the Mycological Society should come i :)
YES YES YES. So much yes. I live in the lush forests of the pacific nw and was hoping there would be some sort of mycology discussions on steemit. I followed your page in hopes of seeing more, as well as some of the users from this thread.
Im relatively new to mycology but have become heavily immersed and educated in a very short amount of time.
Thanks for this. Keep up the great work.
and
Happy hunting!!!
Always happy to see another fungal enthusiast!
There are definitely a group of interested parties here on steemit, although I'm sorry to say I haven't heard from several of my favorite mushrooms nerds recently - @fabulousfungi @elianasgarden where have you gone!? - but lots of others do take an interest in mycology, especially in the homesteading community, where mushrooms are probably encountered more frequently, and sometimes with an eye toward foraging.
I will often cross post my content between here, my website and reddits /r/mycology and /r/slimemolds subs - as well as the slimemoldcollective now and again. All great resources. Also, the links and books that I cite to are also great resources if you want to get a hint for various IDs.
Eventually though it is helpful to learn from more experienced people in person, so get involved in your local mycological society. I'm quite sure there is one near you as the Pacific Northwest is a fungal paradise!
Looking forward to seeing you around!
I just followed the missing mycologists you tagged. Maybe we can bring them out of hiding ;D. Also, NICE SITE. Definitely will keep checking for new content.
I'm a member of the Pacific nw mushroom page on fb. My area is full of chanterelles and I'm working on training my dog to hunt truffles! Also am attending truffle fest here next month.
Feel free to tag me in any articles you think i might enjoy. Always down for nice mushroom read!
Once i make my intro post ill start sharing the crazy fun-guys growing all over my property. I've recently tried cloning turkey tails and they aren't dead yet so i may do a time lapse of that AS WELL as the time lapse of a certain magical variety whose spores i ordered last night ;)
I have not even broached the topic of growing from spore yet, as I've never tried, but I have a non psychoactive coprinus and Agaricus species i want to try with.
You seem to be very knowledgeable and I'm always super excited to connect with a new mushroom enthusiast!
The only thing is I don't ever talk about those "special mushrooms" in my blog, although I'm sure they would increase readership if I did. Given their legal status here in the US, out of an excess of caution, I try not to upvote related content either. I only mention in order to point out that everything we post on steemit is, in theory, immutably and publicly disclosed on the blockchain - so I take a very conservative view on these kinds of issues.
Having said that you will have my eyes, ears and vote on any other mycological content! Really want to see the fungal ecosystem you're living in and very interested in the cloned turkey tails!
Edit: Although to be clear, there are no psychoactive components in the spores of those mushrooms! Only in the final fruiting body.
Edit 2: Also don't mean to scare yiu off by broaching the topic - it's just the permanence and transparency is something to be cognizant about if you decide to go that route content wise.
That was one of my concerns. Not only because i have no anonymity on here, but because forum-trolls looove to ruin peoples non-internet lives. Of course they could be "only for scientific research purposes and promptly destroyed afterwards." :) ive just always wanted to try growing indoor from spores and these certain ones seem to be a very adaptive, resilient strain so i thought itd be a fun first experiment. :)
These fungi really morph quickly from one stage to the other. I just wonder how long the slime mold you were examining at first can remain extant on a piece of old bark.
These certainly transformed very quickly - so far, I don't actually think I've seen a confirmed slime mold on any of my moist chamber barks - this one, even though it looks slimy, is probably a fungus as well.
However, the question remains pertinent - the fruiting bodies of both slime molds and fungi can sometimes last for very long periods of time - with some species many many months. These guys are quite small and in a fairly controlled environment, so I would think there will be evidence of them on the bark for the foreseeable future.
Interesting. I know little about fungi but I thought that most of them are extant for a very limited time (2 weeks at the most). In the San Fernando Valley of Los Angeles were I was born, I would see only two varieties: a slimy brownish colored mold specimen that grew on decayed trees and a white mushroom with a white cap with brown streaks on it; the latter seemed to go away as soon as it appeared.
There's a fair amount of variety in terms of how long the fruiting body lasts - many more conventional looking fungi only last a short time, some only a few days. Some Corprinus species are famous for literally melting into black ooze just a few days after first appearing. Other gilled mushrooms take longer to entirely disappear - while some woody polypores can last, in some form or another, for multiple seasons.
In terms of these little guys there just isn't a lot to disturb them except me and other microorganisms on the piece of bark. In nature rain, wind, snow and other animald would play a role in eliminating signs of the fungal growth. Now I don't know yet how this particular species even spreads it spores - so it could be it disappears as a result of its own physiology in the coming days. Time may tell.
Very interesting really. I always like your posts if I see them.
Further research revealed this probably IS a slime mold! So, from my perspective, that's even more exciting!
I'm with you on the ID challenges, @dber! I see so many fungi that I will never know by name. Sometimes it's even too much to know their relatives. And in the papers that sort out fungi by DNA, the results are mostly downright probabilistic rather than definitive. It's humbling, too, to read older mushroom books - even great ones - and realize how much has changed in 'knowing' what a specific mushroom might be.
And I sure appreciate all that effort you go to, to get photos and footage at such a small scale. I hope you can set up a great lab somewhere, that gives you more room to have temperature control and lighting the way you want! -- And I'll stay away from foraging those slime molds - to eat, anyway..... ; )
Haha - I know it's silly I still have the disclaimer up for these obscure, tiny things - but you never do know what folks might try to eat!
You're absolutely right re:identification - DNA has really stirred the pot so completely stirred the pot that everything is still falling back into place. It's part of the reason I'm so hesitant to eat what I forage, even though lots of factors may support edibility for a given mushroom. End of the day it's just so easy to get it wrong.
I'm gonna be working on some more "laboratory" upgrades as the year progresses - just need to figure out where I'm gonna put them ... :)
May I be nasty? They first looked like a lychee. Then next looked like a burned lychee... :D
Hah - you certainly may, the slime mold won't mind. Especially the one I vivisected :)
At first I didn't understand what was happening in the timelapse video. What am I supposed to be observing? All I see is the light moving away for some reason.
And then I read further. Wow!
The spores remind me of lentilles du Puy!
The Exidia glandulosa looks like something the dog left on the lawn! :P
When shall we be expecting (how much more steem do you need to buy) a scanning electron microscope?
Thumbs up for the last two paragraphs (about moral and intellectual integrity). 👍
Funny thing, almost right after I finished writing this and posted it I found the new Slime Mold online resource I'd needed before and almost certainly ID'd these things - but I tend to prefer keeping my mistakes, or imperfect content, visible, but corrected - rather than indulge in essentially the post-facto illusion of clarity. The whole things a process :)
wow very impressive pictures
What a spectacle, what nature is !!!
Excellent images, good information.
Regards...
there are many kinds of mushrooms, in my place many are poisoned by eating them.
there is a kind that grows in straw and burials that can be eaten. most others can kill us.
thanks for the information @dber . mushrooms are cute little time, but horrible when it's big,
There are definitely a lot of dangerous mushrooms out there!
I'd love to see your entry for my contest to win 5 SBD!
https://steemit.com/contest/@anthonyj/usd5-sbd-artist-photographer-500-follower-giveaway