To celebrate SteemSTEM’s launch of their new website (https://steemstem.io), I’m writing a series of posts on the recently announced 2018 Nobel Prize in Chemistry. This year’s prize was awarded to Frances H. Arnold for pioneering the first directed evolution of enzymes, and to George P. Smith and Sir Gregory P. Winter for developing phage display 1. The award was a major story in a bunch of news outlets, but I found most of the pieces were scant on details. They only gave a cursory overview of the techniques that the scientists spent a major portion of their lives developing. Fortunately, @SteemSTEM has given me the platform to remedy that problem. Each entry in this series will focus on one of the scientists’ contributions, try to explain that contribution and show why the scientist deserves this prize. Today’s focus: George P. Smith and Phage Display.
Who is He?

George P. Smith (2).
He genuinely looks like a happy guy :)
George P. Smith has an impressive, albeit unexciting academic career on paper. He got his Ph. D in Bacteriology and Immunology at Harvard University, took a postdoc at the University of Wisconsin, and then became a professor at the University of Missouri in 1975 3. For the next 43 years, he continued to be a faculty member at the University of Missouri and is now a Professor Emeritus. This position usually refers to a semi-retired professor that is still given university resources to pursue his or her research interests, but doesn’t have the responsibilities of a full professor (all professors, whether they know it or not, strive to become a Professor Emeritus and then die at the bench). George Smith’s current research focuses on using his phage display technology to visualize cancer, and he still publishes every few years.
All in all, George P. Smith’s career might have been unremarkable if not for the magic of sabbaticals. A sabbatical is a paid leave of absence for professors, usually lasting a year and taken once for every seven or so years worked. Back in the pre-internet era, this was a key opportunity for academics to broaden their horizons. They could learn first-hand from other specialized labs and bring that specialized knowledge to their university. Sabbaticals are a bit less important now that we can share information so easily, but they still provide an excellent opportunity to work with resources and experts that might not be available at a professor’s home university. Some professors blow the opportunity off, but George Smith knew exactly what he wanted to do. He secured a spot in Robert Webster’s lab at Duke University which had the equipment and know-how accomplish his goals and got to work 4. You could argue that George P. Smith had the greatest sabbatical in history as he used it to develop a revolutionary technology that cemented his position in science history.
Oddly enough, this isn’t the first George Smith to win a Nobel prize. In 2009, George E. Smith won the 2009 Nobel Prize in Physics for helping develop digital imaging technology integral to modern digital cameras (https://www.nobelprize.org/prizes/physics/2009/smith/facts/). This isn’t important, but it made researching this guy’s bio a heck of a lot harder so I needed to vent about it.
What did he do?
The technology in question is known as phage display, a technique designed to improve the utility of directed evolution. The process of directed evolution was developed by Frances Arnold, the subject of the previous post in this series. In brief, directed evolution was a rapid way to generate thousands of protein variants, and completely changed the way scientists could improve upon proteins. However, it still had a major weakness in that it didn’t provide an easy way to analyze the created protein variants. In Dr. Arnold’s original paper, she used a specialized protein with a unique method of assessing activity, but that method doesn’t apply to proteins in general. In fact, most proteins stay inside a cell and are difficult to analyze without breaking open the cells they’re in. Not only is this time consuming, but it’s hard to keep track of the cell that a particular protein variant came from.
Dr. Smith circumvented this problem by genetically engineering bacterial viruses (known as phages) to express the proteins of interest on their outer coats allowing for them to be easily assessed or enriched. It was called phage display because the phage is exposing or “displaying” these proteins to the external environment. His initial work on developing this technology can be found in the science publication Filamentous Fusion Phage: Novel Expression Vectors That Display Cloned Antigens on the Virion Surface 5 and this article does a great job of laying out the groundwork for what would become known as phage display.
Phage display works by fusing a gene of interest into the middle of a viral gene that normally exports to the surface of the phage. Dr. Smith choose filamentous phage gene III which has a distinct signal peptide sequence.
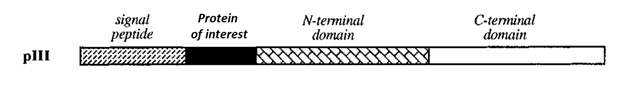
Diagram of the protein of interest fused within the filamentous phage protein III 6.
When this signal peptide is synthesized, the virus exports the attached protein to the outer coat of the phage. When the protein of interest is fused between the signal peptide and the rest of the protein, it will wind up on the outside of the virus with the rest of the protein tethering it to the outer coat.
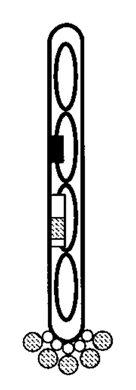
The protein of interest (hatched circles) is exported to the outside of the phage and stays attached by a portion of the original protein (white circles) (6).
The protein will stay firmly attached to the phage, but can be exposed to external stimuli. This exposed-protein attached to a virus arrangement opens up a lot of opportunities for scientists. The most obvious to Dr. Smith (and generally the most effective use of this technology) was affinity purification. That is, scientists can use phage display to isolate proteins that bind to a specific substrate of interest (like an antibody), then propogate the phage to amplify the proteins that bind the strongest.
The article makes it clear from the start that this is a proof-of-concept paper. Dr. Smith’s main goal is to show that his idea of phage display works in practice, so he started with a model protein. That protein is the EcoRI endonuclease 7. This is a well-studied protein that cleaves a specific DNA sequence (GAATTC to be exact). More importantly, antibodies specific to this protein are readily available so affinity purification is relatively easy. Dr. Smith artificially created a mixture in which phages displaying EcoRI were vastly outnumbered by a control phage (phage m13mp3). The specifics of why the control phage was chosen is a bit beyond the scope of this article, but suffice to say it can easily be quantified and can be distinguished from the EcoRI displaying phage.
The exact steps of the experiment were as follows: Dr. smith adds a few EcoRI phages in a big mixture of control phage and spreads the mixture onto a petri dish with affixed antibodies specific to EcoRI. The mixture is allowed to incubate so that all the EcoRI displaying phages bind to the affixed antibodies. The plate is then washed multiple times which removes the control phage while the bound EcoRI displaying phages remain. Finally, the phages that remain bound after multiple washes are eluted and the amount of control phage and EcoRI phage relative to the initial phage input are recorded.

Affinity purification of phage with an EcoRI gene fusion from control M13mp8 phage 5.
In the results shown above, you can see that the washes removed almost all the added phage, with less than 0.5 % of the total phage added remaining. However, the amount of EcoRI phage that remained was vastly higher than the amount of control phage remaining. In this particular experiment it was enriched by 3400. Thus, the affinity purification was successful which confirms that Dr. Smith’s concept of phage display works in practice.
Why does this deserve the Nobel Prize?
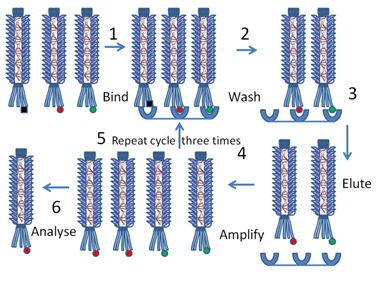
Diagram showing how phage display can be used to isolate and analyze the red protein of interest (8).
It’s also important to note here that Dr. Smith didn’t stop his work on phage display with this single paper. He continued to push this technology forward and make it more and more useful. In this sense, the Nobel prize recognizes a lifetime of achievement rather than just one lucky finding during a sabbatical.
There are many, many applications for phage display. From basic research purposes like receptor identification to more applied functions like drug discovery or vaccine development 6. Next week, I’ll be delving into one major way in which the technology of phage display was adapted to create antibodies for therapeutic uses. This work was the product of Gregory P. Winter, the third and final recipient of this Nobel prize.
References
(1) https://old.nobelprize.org/che-press.pdf
(2) https://biology.missouri.edu/people/?person=94
(3) https://en.wikipedia.org/wiki/George_Smith_(chemist)
(4) https://medschool.duke.edu/about-us/news-and-communications/med-school-blog/nobel-prize-winner-george-p-smith-completed-early-award-winning-research-duke
(5) http://science.sciencemag.org/content/228/4705/1315
(6) http://www.biosci.missouri.edu/SmithGP/PhageDisplayWebsite/PetrenkoSmithChemReviews.PDF
(7) https://www.neb.com/products/r0101-ecori#Product%20Information
(8) https://en.wikipedia.org/wiki/Phage_display
Images
All images were taken either directly from the publication referenced or labeled for reuse on Google Images. George P. Smith’s photo was taken from the University of Missouri’s faculty page. If any image owner has an issue with this article, please contact me and I will address the issue.

This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io and @curie.
If you appreciate the work we are doing then consider voting all three projects for witness by selecting stem.witness, utopian-io and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Appreciate vote and all the efforts of the curation team as always.
I guess all of us scientists secretly wish for our own death-bench XD
Great post!!!
Stop people, I'm losing my breath
Thanks for reading!
I may be the counter example: I don't wish for my own death (... yet).
Lol
Great Article T. Despite having worked at a place that loves phage display, I know very little about it. I knew even less about George P. "Sabbatical King" Smith.
Hah! Same here even though my PI loved using phages and we had someone on staff that was good friends with George Smith and extensively used the tech.
With such a name, I am not surprized he is not the only one ;)
Yeah there are a lot of people called Sabbatical King. :D
That sounds like a bad burger :D
Made out of lab grown meat.
If only it was tastier (and healthier)...
Am not so much of a chemistry fan but I did love reading this article. It makes me know that we could all be someday Nobel Prize winners while still on vacation.
Hah! Well this guy did devote most of his career to phage display after his sabbatical, but I suppose you could call it a vacation of sorts.
LOL :D the best sentence I read today.
The rest of the text is also great
Ha! Glad to see my attempts at humor sometimes succeed :p
My fate is thus sealed :D
Thanks a lot for this series. Without it, I think that I would have never read anything about the Nobel prize in chemistry. For some reason, this part was much easy to get than the first one (although both were pleasant to read, don't worry), and I have no question (except the jokes I have left through the other comments)!
Thanks. It’s very useful to hear that this entry was easier to understand so I appreciate the comment. I’ll have to keep in mind what I did to make it Moreno accessible.
Just updated my above comment: Nobel prize in chemistry, not physics :D
The research has nothing to do with chemistry anyways so the mistake is understandable :P
But chemistry is the most reasonable field in which to classify those researches, isn't it?
I suppose you could make an argument for this belonging in Physiology or Medicine, but chemistry often goes to molecular biology discoveries so its a bit of a toss up.
I won't argue with the Nobel committees... ;)
Hi @tking77798!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Once again, another post upvoted by curie that triggers the inner science geek I used to be (let's just keep that side toned down). It's funny because I can understand the technical stuff mentioned here when it's these topics I find myself avoiding. A fun informative read and commendable :D
You shouldn't bury your inner science geek. That's what curie and steemstem are all about!
Regards. My native language is Spanish. I use online translators to better understand the publicatio.
I am very curious to read this article.
The subject seems to me to be of microbiology or virology. No chemistry. However, the prize was in chemistry.
Even so congratulations....
FROM VENEZUELA
Yeah, this work (and the work of the other chemistry nobel laureats this year) is much more microbiology focused than chemistry focused. Thanks for the support!
Greetings from Venezuela
perfect
please support me as well
@dominicbaitan/manage-diarrhoea-yourself-description-of-drugs-used-in-diarrhoe
Hmmmm....
The world of protein variance is amazing, and each new discovery or workaround of it just gets even more interesting.
I remember the first time I studied biochemically how complex the process of protein formation from DNA can be, and how (in theory) could be engineered, plus experimental trials on it. Really good to see some techniques on it!
Thanks for bringing it to our attention @tking77798!
Thanks for the synopsis @Deholt
Thanks for the synopsis @Deholt
Congratulations @tking77798! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board of Honor
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Congratulations @tking77798! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board of Honor
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard: